While pharmaceutical companies have proven the return on investing in digital at early stages of drug discovery, manufacturing has been slower to embrace digital transformation.
To understand how pharma R&D and manufacturing can unlock more business value through adopting digital tools and technologies, we speak to Digital CMC consultant Mark Buswell.
Mark is a chemical engineer by training, with over 20 years in R&D and Manufacturing at GSK, before joining NGT BioPharma consultants to head up Digital CMC services to help pharmaceutical companies navigate digital transformation of their CMC function.
Listen the full interview here:
Digital CMC – a definition
Digital CMC refers to the application of digital technologies in managing the chemistry, manufacturing, and control aspects of pharmaceutical production. It encompasses the use of artificial intelligence, data analytics, automation, and cloud-based systems to optimise the entire lifecycle of drug development and manufacturing.
Digital CMC maturity framework
Mark and NGT Biopharma Consultants have developed a Digital CMC maturity framework, highlighting the steps on the journey towards increasingly transformative business value.
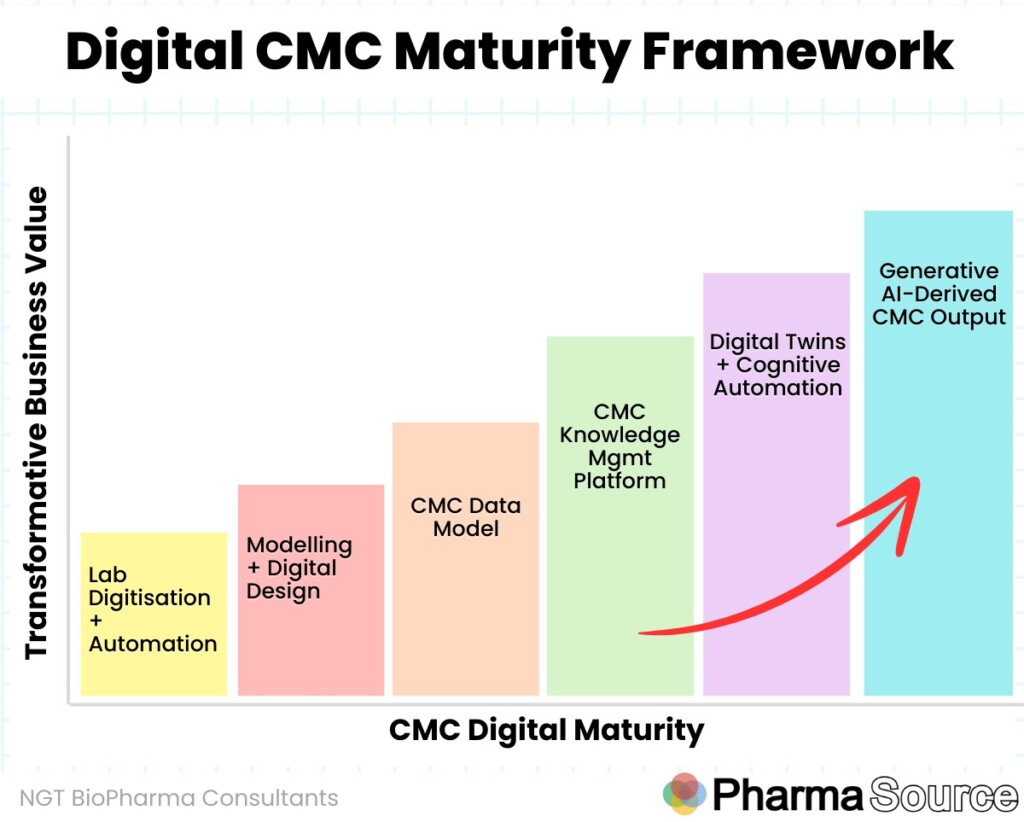
The six stages of CMC digital maturity are:
- Lab Digitisation and Automation
- Modelling and Digital Design
- CMC Data Model
- CMC Knowledge Management Platform
- Digital Twins + Cognitive Automation
- Generative AI-Derived CMC Output
“While it looks linear, there are many different pathways and combinations through this. While the capabilities build off each other, each level doesn’t need to be 100% in place before the next levels can be exploited for specific CMC outcomes” says Mark.
Benefits of adopting digital CMC
Mark highlights four key benefits of adopting digital CMC capabilities:
- Accelerating development of late phase drugs
One of the major benefits of digital technologies is accelerating the development of portfolios through later phases.
“Most investments that have gone into the front-end of the discovery, with investments in genetics, functional genomics, and Machine Learning generating a significant increase in the number of targets that we can go after. Those portfolios of assets are maturing now and moving into the CMC space, so there’s a real opportunity to get those medicines to patients, faster. ”
- Developing more robust medicines using digital technologies.
Mark explains that the use of simulation technologies leads to enhancements in the quality and the design of the drug products.
- Unlock digital efficiencies
“CMC functions are under pressure as margins are get tighter, and have to do more with less. If you want to still deliver the same amount of output, without the quality of the CMC outcomes deteriorating, it’s going to require digital solutions unlock efficiencies.”
- Disrupt or avoid being disrupted
Finally, Mark highlights there are emerging biotechs and startups already utilising this technology, which gives them more opportunities to disrupt slower moving incumbents.
“Smaller ‘greenfield’ companies are finding it easier to invest in modern, sophisticated systems because they don’t have this legacy brownfield of systems.” says Mark
“Large pharma companies tend to get locked into systems for a fairly long period of time, and then it’s quite difficult for them to re-platform. You’re talking about 3-5 years to deploy it, and then you’re going to live with it for next 10 years – it’s not easy to change that midway through.”
Overcoming barriers to change
“Culturally we’ve got a long way to go” before digital is embedded at a leadership level in CMC, say Mark.
“There’s a leadership capability journey that that we need support CMC leaders through. Most would struggle to access modern data platforms. Most CMC decision-making still being done off static PowerPoint slides which are out of date by the time they are reviewed by management.”
“Part of the reason is R&D has had so much success with adopting digital technologies, it that it’s a function very comfortable with managing risk.” say Mark. Afterall, only one in a thousand compounds that go into development will actually make it through to commercialisation.
In contrast, manufacturing is all about operational efficiency uptime and managing operations to very tight margin. “That generates a healthy scepticism in manufacturing decision makers around whether investments in technology are going to pay back or not. The burden of proof for Return On Investment is much higher in the manufacturing space than it is in the R&D space.”
The fact that CMC isn’t broken in its current form is a factor holding it back from digitisation.
“CMC is very rarely on the critical path of drug developments. Generally speaking, if our discovery colleagues can get all the, you know, the efficacy and the safety, correct and get the right targets and the right modality, generally, CMC will deliver a safe and efficacious medicine.”
This can cause organisational push-back, with an attitude that “It’s worked for the last 50 years, why do we need to change it?”
Advice for driving change: Make it specific and real
To drive change, Mark’s number one advice is always to start with the specific business context, and to focus on where the most value can be created.
“For any CMC department. I would always start with the pipeline and the most important work that has to be executed.” he says.
“Always anchor your digital investments in your portfolio, and the closer you can get to specific assets the better.”
“Make it real by picking a specific assets, and explain how digital will make an impact on work, submissions over the next five years. Draw up that timeline and show the opportunities for investments to make a difference. You start to get real about the challenges, and how some of the technologies on the maturity curve can make a difference.”












 Stay ahead of trends and best practices
Stay ahead of trends and best practices
