Paolo Giudici of Johnson & Johnson reveals: “We realised that to remain competitive and aim to be the best supply chain in the world, we needed to start a digital transformation journey. The external manufacturing piece isn’t just part of this transformation—it’s fundamental to our future success.”
Paolo Giudici is part of the Partnerships and External Supply Digital programs team at Johnson & Johnson, bringing over two decades of experience across technical transfer, CMC leadership in R&D an commercial, and supply chain management. His career spans both small and large molecule manufacturing, including a pivotal role in J&J’s COVID-19 vaccine task force, where he established critical manufacturing partnerships during the pandemic response.
In this interview with the PharmaSource podcast, Paolo shares insights from J&J’s four-year digital transformation journey, discusses the pressing need for industry-wide standardisation, and reveals valuable lessons from the COVID-19 response that could reshape pharmaceutical manufacturing partnerships.
COVID-19: A Catalyst for Change
The pandemic response demonstrated the pharmaceutical industry’s latent capability for rapid action. Paolo played a crucial role in establishing new CMO relationships for J&J’s COVID-19 vaccine Drug Product manufacturing, working under unprecedented time pressures.
“It was very time-critical,” Paolo recalls. “We were supplying the vaccine to a very large base of population and had to quickly set up manufacturing capacity. We had to leverage our previous network strategy while reaching out to a broader base of CMOs.”
The experience highlighted the industry’s ability to compress timelines dramatically. “We’ve seen processes that typically took multiple years being done in matters of months,” Paolo notes. “The regulators’ approach in certain conditions was a significant factor, but equally important was the CMO industry’s ability to make capacity available quickly.”
Comprehensive Digital Transformation in External Manufacturing
J&J’s digital transformation program encompasses their entire external manufacturing ecosystem, from chemicals and small molecules to large molecules, advanced therapies, gene therapy, and CAR-T. The initiative focuses on several key areas:
1. Transactional Integration
“We’re establishing secure connections between CMO and sponsor ERP systems,” Paolo explains. “This creates end-to-end visibility from orders and forecasts through to inventory management, batch tracking delivery, and potentially payment processing.”
The objective is a connected ecosystem similar to the following diagram:
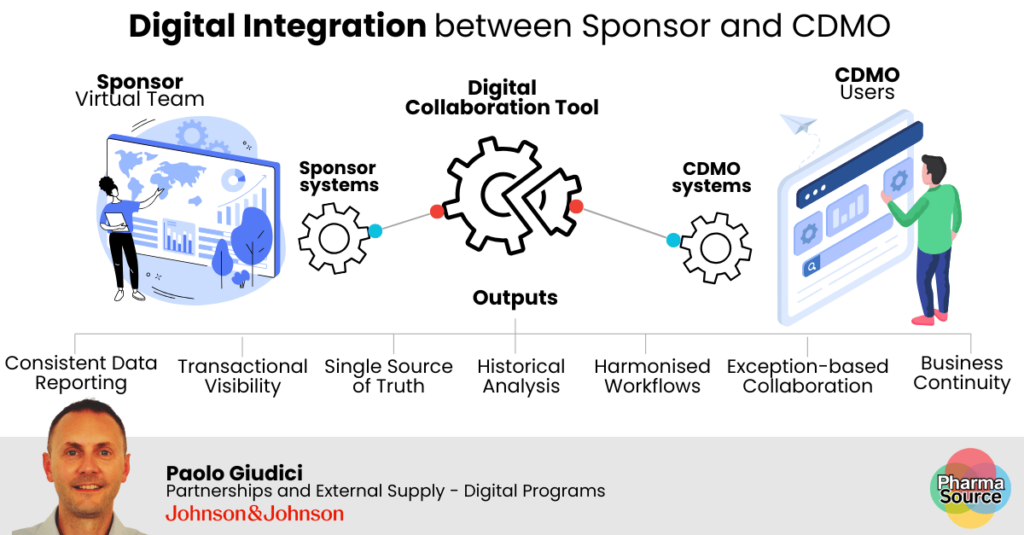
2. Manufacturing Floor Visibility
“Particularly in biologics and large molecule drug substance manufacturing, we’ve implemented real-time visibility of some of our shop floor external operations. This allows experts to monitor processes remotely, create predictive models, and intervene proactively if processes begin to drift outside acceptable parameters.”
3. Quality Management
“The quality aspects of digital collaboration are crucial. We’re working to standardise how quality data is shared and monitored across our partner network.”
The Standardization Challenge
Paolo identifies standardisation as perhaps the most critical challenge facing the pharmaceutical industry’s digital transformation. Unlike mature industries such as automotive or aerospace, pharma lacks standardised processes for digital collaboration between sponsors and CMOs.
“Currently, if a sponsor attempts to establish digital collaboration with a CMO, they essentially start from scratch,” Paolo explains. “There’s no standardised framework for what data should be shared, how it should be formatted, or what systems should be used.”
This challenge is amplified by the multiple platforms currently in use. “CMOs are facing different clients with different technology requirements,” Paolo notes.. This creates significant complexity for CMOs who must manage multiple platforms and login requirements.”
To address this, J&J is working with industry associations like Bio Forum to develop standardised approaches. “We’re working on creating a playbook – a thorough roadmap for how digital collaboration should be set up,” Paolo reveals.
Implementation and Change Management
Success in digital transformation requires careful attention to change management and implementation strategy. Paolo emphasises several key recommendations:
1. Establish Common Intent
“Before diving into change management and communication, there should be a contract in place for digital collaboration, or at least a written agreement that both companies share basic intentions to pursue digital collaboration.”
2. Focus on Value Delivery
“There’s often scepticism or fear that automation will eliminate jobs. The reality is that skills are evolving, not disappearing. Digital collaboration will automate repetitive tasks, freeing people to focus on more strategic initiatives.”
3. Cost Sharing
“The cost aspect requires careful consideration. Typically, certain basic costs are borne by the sponsor pharma companies, while system-specific costs are taken by the CMO. This needs to be clearly agreed upon upfront.”
4. Industry Collaboration
“Connect with companies who have already begun this journey. Many large CMOs have experience with these types of programs now. It’s crucial to reach out, discuss experiences, and engage with industry associations.”
Future Outlook
Looking ahead, Paolo sees digital capabilities becoming increasingly central to CMO selection. “Digital capabilities will be evaluated alongside traditional criteria like capacity, quality systems, and reputation,” he predicts. “CMOs and (bio)pharmaceutical companies need to prepare for this evolution to remain competitive.”
J&J is now focusing on consolidation and standardisation. “We’re in a consolidation phase with our existing technology deployments,” Paolo explains. “Simultaneously, we’re working with other pharma companies and CMOs to move towards industry-wide standards for digital collaboration.”
Paolo concludes with advice for organisations beginning their digital transformation journey: “Be conscious that this is a long journey. Don’t expect fast execution, even though the program should be time-bound. Perform proper technology scouting and make careful selections of technology partners. The future of pharmaceutical manufacturing digital collaboration with CMOs depends on getting this right.”
Paolo Giudici will be sharing more insights at CDMO Live in the AI and Digitisation of Contract Manufacturing panel, alongside Dave O’Gara – sponsored by Aizon. Find out more










