“Treat them as a partner, not just a supplier. It’s a mindset you need to get into.” – Federica Fraschetti, MSD
Federica Fraschetti, Associate Director of External Manufacturing at MSD, brings years of experience in the pharmaceutical industry and a passion for optimising partnerships with contract manufacturing organisations (CMOs).
In the latest PharmaSource podcast episode, Federica explains why getting supplier relationship management right is a key strategy for driving efficiency and fostering innovation in the biopharma industry.
Building a Virtual Plant Team
Creating an effective CMC operating model is crucial when working with external partners. Federica emphasises the importance of a clear internal structure that is visible to CMOs:
“What is really key is the structure that you have internally and then externally. So what the CMO can see of your structure when dealing with partners,” she explains.
MSD utilises a ‘virtual plant team’ model, which includes representatives from key functions such as operations, technology, quality, supply chain, and procurement. This structure provides:
- Clear supporting model for day-to-day operations with the CMO
- Established escalation paths
- Defined roles and responsibilities
While it’s ideal to match these roles with counterparts at the CMO, Federica acknowledges that this isn’t always possible. “It doesn’t mean that each function needs to be one-to-one matched at the CMO, but you do need to know where it is matched, even if it is the same person or the same function,” she advises.
Establishing Effective Collaborations with CMOs
Federica presented a framework for working with contract manufacturing organisations:
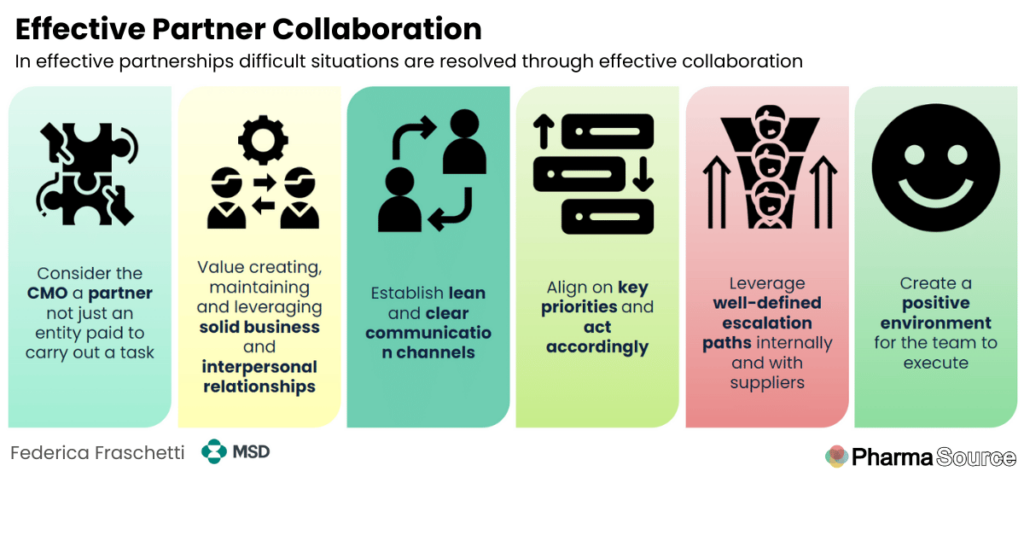
- Treat CMOs as partners, not just suppliers: “Do not treat this CMO just like a supplier. I’m paying for a service, I’m getting the service, and this is it. There is a lot more to that,” she emphasises.
- Build personal relationships: “It really helps to pick up the phone and have an informal conversation on what you need, why you need it, and why it should be prioritised,” Federica advises.
- Establish clear communication channels: Define which functions interact with which counterparts at the CMO to streamline communication and escalation paths.
- Set and act on clear priorities: “If I have two products, and I say product A is priority, I need to act accordingly,” she stresses.
- Use escalation paths constructively: “Escalation should be looked at as the team has done everything they could working together to resolve this issue, but we just couldn’t get what the program needs,”
- Creating a Positive Partnership Environment. To foster a productive partnership, especially when working remotely or with international partners. Tips to create a positive environment include:
- Maintaining transparency in all communications
- Avoiding finger-pointing and focusing on constructive problem-solving
- Meeting face-to-face regularly, not just when issues arise
- Utilising shared digital tools like SharePoint for efficient document management
The Critical Role of Quality
Quality assurance plays a vital role throughout the lifecycle of a drug, but its involvement can vary depending on the project phase. Federica strongly recommends involving quality early in technology transfers:
“My advice is to get quality involved early on in technology transfers. That doesn’t mean that they need to approve all of the documents. That just means they need to be informed why decisions are taken and why studies are designed in a certain way.”
This approach helps to ensure quality is up to speed with the project history so that any potential gaps can be addressed before they become a problem.
Stay agile
The biopharma industry is experiencing a push for faster clinical program development. Federica notes, “The major trend I’ve been observing is that my company, and probably other companies as well, are trying to adapt their structures to be able to achieve that.”
This shift requires companies, especially larger ones, to adapt processes and ways of working with Increased agility in project management.