
Terumo is a mid to large size-global medical device company based in Japan, with offices in over 160 countries around the world and over 50,000 products and services.
The Terumo Pharmaceutical Solutions Division, also known as ‘PSD’, is a leading manufacturer of injectable solutions – including primary container, infusion therapy devices, general drug delivery devices – and contract development manufacturing (CDMO) services for parenteral drugs.
“2024 so far has been very good for us.” says Marco Chiadò Piat, President of Terumo Pharmaceutical Solutions Division.
“We’re seeing continuous growth this year, as we did in 2023 and 2022. Continuous growth in capabilities, the solutions we provide to our pharmaceutical customers, and hence a continued increase of the positive impact that we have on patients worldwide.”
Ahead of CDMO Live, we sat down with Marco and Anil Busimi, Vice President Strategy & Marketing at Terumo Pharmaceutical Solutions Division, to discuss the integrated CDMO services that Terumo offer to their growing, international customer base.
Doubling capacity
“The trend to outsourcing of injectable drugs is expected to increase.” says Anil. “Many pharma companies are deciding to reduce the risk and increase flexibility by outsourcing.”
Terumo have three plants in Japan, in Fujinomiya, Kofu, and Yamaguchi.
Last year Terumo announced a 52.2 Billion JPY ($337 Million USD) investment in their CDMO capabilities with a new facility in Kofu, Japan. Scheduled for completion in 2025, the project will double the company’s capacity and feature automated fill and finish equipment and device assembly equipment.
Anil explains how Terumo support customers around the world. “Even though our facilities are Japan, we service global markets and have experience going through the audits of regulatory agencies such as the FDA or EMA, to help customers launch their product on a global scale.”
Integrated end-to-end CDMO services
“Terumo have been operating their integrated CDMO business model for almost 20 years. We combine a range of services into one single partner” says Marco.
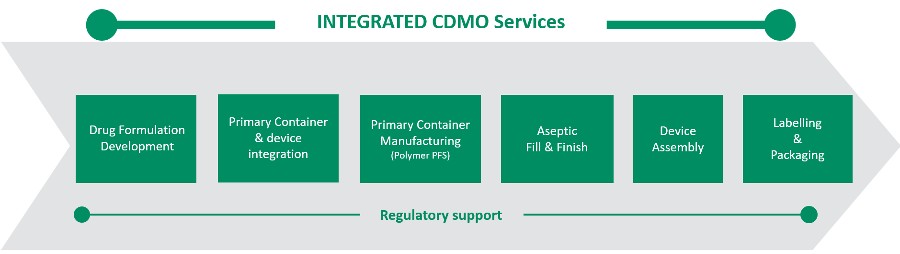
“From providing primary containers such as polymer pre-fillable syringes, or customised containers, we carry out the drug device combination development itself, we perform aseptic fill and finish, and we can assemble the prefilled syringe into auto-injectors or additional safety devices.”
“In addition, we provide our customers with regulatory support, and we accompany them to a commercial stage. “
“Our integrated capabilities help our customers to reduce the risk of the project, reduce the time to market. More importantly, if you also look at it from a sustainability point of view, doing all the operations on one site leads to a lower carbon footprint.”
“I think we are the only player on the market where all these elements of these operations, especially the primary packaging / fill and finish come together in a single ‘one stop shop’.” he says.
“Customers can come to us with an API and actually walk out with a final drug product, which is ready to deliver the drug to the patients,“ says Anil.
The division’s focus is on injectable drugs, particularly biotech/biosimilars at phase two or phase three. “Given our capabilities and our understanding of the primary packaging, and drug container interactions, we are well placed to support silicone sensitive drug molecules.”
“Most of the customers are also targeting to bring those therapies from a hospital to home environment. That means combining those prefilled syringes with an auto injector or with a safety drug delivery device. We can help them launch a finished drug product, which is ready to be injected by the patient.” says Anil.
Terumo’s drive to Net Zero
“Sustainability is a very important topic for healthcare, and we all need to contribute.” says Marco.
Terumo is committed to Science Based Targets of reduce absolute GHG emissions by 50% by 2030. The company will increase a ratio of renewable electricity use up to 50% by 2030 and aims to achieve carbon neutrality by 2040.
“It’s important to underline these are not just corporate goals.” says Marco.
“There’s a significant amount of people working on this, not just a small department. We are doing this not just for the environment or our pharmaceutical customers, but also for each and every one of our families.”
To find out more about Terumo Pharmaceutical Services, make sure you meet them at CDMO Live on June 13th 2024. Register today for your free ticket.












