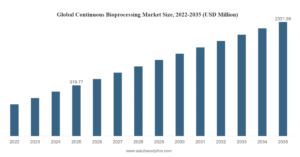
The Indian CDMO market is forecast to double over the next five years, as ‘the pharmacy of the world’ becomes increasingly attractive for contract development and manufacturing outsourcing.
To understand what is behind this rapid growth, we spoke to Akhil Ravi, Chief Executive Officer Aurigene Pharmaceutical Services, the contract research, development and manufacturing organisation arm of Dr. Reddy’s Laboratories.
Akhil explains that India is emerging as a ‘strategic hub’ for biopharma outsourcing due to a confluence of factors that appeal to global pharmaceutical and biotech companies for a number of important reasons:
“India has an abundance of well-qualified professionals, including scientists, researchers, and technicians, at a fraction of the cost compared to Western countries. This cost-effectiveness also extends to infrastructure and operational expenses, enabling biopharma companies to save significantly.”
“India has a well-established network of manufacturing facilities compliant with international regulatory standards, such as the US FDA and the European Medicines Agency.”
“Most Indian pharmaceutical companies are digitizing their processes, ensuring data accuracy, compliance with regulatory standards, and enabling advanced analytics for proactive quality control. Quality standards ensure the efficacy and safety of medicines for patients; being patient-focused, the industry should always continue to raise the bar further”.
Akhil explains that India has the second-most USFDA-approved manufacturing plants outside the US and remains the largest global supplier of affordable medicines.
“The Indian government has also proactively supported the biopharma sector through favourable policies, tax incentives, and investments in biotech parks, further incentivizing foreign investment and partnerships.”
“These advantages and a strong commitment to quality and innovation position India as a strategic hub for biopharma outsourcing.”
Growing capabilities in biologics
India is well-known for small molecule manufacturing, but have significantly smaller market share in emerging biologics and cell and gene therapies.
Akhil explains that India is “already advancing rapidly in biologics manufacturing”, pointing to how companies such as Dr. Reddy’s have a two-decade track record of releasing biopharmaceuticals to highly regulated global markets.
“On the cell and gene therapy side, we already see ImmunoACT’s NexCAR19 CAR-T cancer therapy being manufactured in India at around 10% of the cost of comparable products available globally, and companies such as Miltenyi placing centers of excellence in India to support the rapidly developing CGT market.”
Contributing drivers to these fast-growing sectors include experience in cGMP manufacturing of complex vaccines, a vast and highly trained STEM workforce, increasing access to capital, and growing realization of significant economic advantages, especially in light of the pressure that the US Biosecure Act is putting onto the Chinese CDMO marketplace.”
Despite this, India does currently lack a “significant domestic innovative biologics discovery ecosystem” which brings challenges, says Akhil.
“Despite tremendous growth, India still needs more experienced management and operations personnel in the cGMP manufacturing space – a challenge that is being faced globally. This gap is being partly addressed via the repatriation of significant numbers of highly trained personnel from the US and Europe. Finally, some domestic regulations could be revisited to facilitate the more rapid transition of development candidates into cGMP production and onwards into vital first-in-human clinical development.
What will be the main driver of growth for the Aurigene?
Aurigene’s growth strategy is focused on strategic partnerships driven by excellent customer experience, ensuring long-term loyalty and collaboration, says Akhil.
To achieve this, they are developing new capabilities and technology platforms to expand our offerings and meet growing market demands effectively. This includes:
Increasing capacity
“To reduce the time to market for a molecule, we are building new capacities and upgrading existing facilities. We have developed a reasonably aggressive capital investment strategy to extend our R&D and GMP manufacturing capabilities for small and large molecules, including new modalities. We are investing heavily in upgrading our existing labs and setting up dedicated manufacturing lines within our existing footprint.”
“Recently, we operationalized a new biologics facility spanning 70,000 sq. ft. in Genome Valley, Hyderabad, India. This state-of-the-art facility specializes in process development, analytical services, and small-scale antibodies and recombinant proteins manufacturing for preclinical and early clinical phases. Operational laboratories are already active, with full manufacturing capacity expected by the end of 2024. This expansion will allow us to provide our customers with seamless services from discovery to commercial manufacturing.”
Digitisation
“We are moving towards 100% digitization of our operations. Recently, we introduced Aurigene.AI (an AI-assisted drug discovery platform) and DEL (DNA Encoded library) platforms to reduce the hit-finding timelines.”
Sustainability
“As an environmentally responsible company, we continue delivering on our ESG goals. We became water-positive in 2023 and are working on ambitious goals to reduce our carbon footprint. In numbers, we aim to switch to 100% renewable power by 2030 and achieve carbon neutrality in our direct operations (Scope 1 and Scope 2 emissions) by 2030 and a 12.5% reduction in indirect carbon emissions across our supply chain (Scope 3 emissions) by 2030.”
Talent
“As a Dr. Reddy’s Laboratories company, Aurigene Pharmaceutical Services has a very good reputation among scientists, which attracts talent. ” says Akhil.
“More than 90% of our workforce are scientists, many of whom have Ph. D.s and Postdocs from global universities. We provide a variety of development programs on the job that help individuals advance from individual contributors to leaders. When we bring in new graduates, we have a six-month flagship program called ‘Prarambh’ that helps their transition from academic to industry.”
Read more about the growth expectations for the India CDMO market and download this infographic here