Anirban Mukherjee, Head of Procurement at Immunovant, argues that while Supplier Relationship Management (SRM) is crucial for achieving high performance in complex industries such as pharma and biotech, it often receives insufficient attention.
Supplier Relationship Management is a ‘systematic approach to evaluating vendors that supply goods, materials, and services to an organization. It involves determining each supplier’s contribution to success and developing strategies to improve their performance’.
In the latest episode of the PharmaSource podcast, Anirban explains the steps to take when implementing a successful SRM programme, drawing on his experience at Ford, Becton Dickinson, and Gilead, where he was responsible for outsourcing to Contract Research Organisations (CROs).
Listen below or wherever you get your podcasts.
Anirban is passionate about SRM driving business outcomes: “Over the course of your career, you realize what you deeply believe in” he says.
From his time at Ford, he learned a “very structured methodology of thinking about how you plan for operational delivery and a structured problem-solving methodology that sort of gets ingrained into your DNA.”
Assessing your organisational maturity
The success of a Supplier Relationship Management rollout hinges on the maturity journey of your internal organisation, says Anirban.
“SRM solutions have to match what your business needs at that time—and that will change over time.”
“In a typical five-stage category management process, the early focus lies on sourcing operations (including RFPs, negotiations, supplier selection, contracting, and purchase orders). Only once you gain a good handle on that, can you start to move upstream with category strategies and market intelligence or risk management.”
Anirban’s advice is to “take a holistic view of your maturity model. If the business primarily seeks sourcing operations, it might not be the ideal time to embark on a full-blown SRM journey”
Educate business leaders
He emphasises that suppliers don’t merely belong to the sourcing team; they belong to the entire business. “While the sourcing team acts as a bridge, it cannot be the sole entity investing time and effort” he says.
Anirban advises assessing the internal education level of executives regarding the importance of dedicating time to engage with suppliers.
“Often, executives become involved only when a critical issue arises. Making a compelling case and demonstrating the value of investing time in supplier relationships is essential.”
Supplier Stratification
The first step toward a successful SRM program rollout is supplier stratification, segmenting suppliers based on a defined set of criteria
Anirban highlights the need to slice and dice suppliers strategically, sharing this matrix to help define the approach:
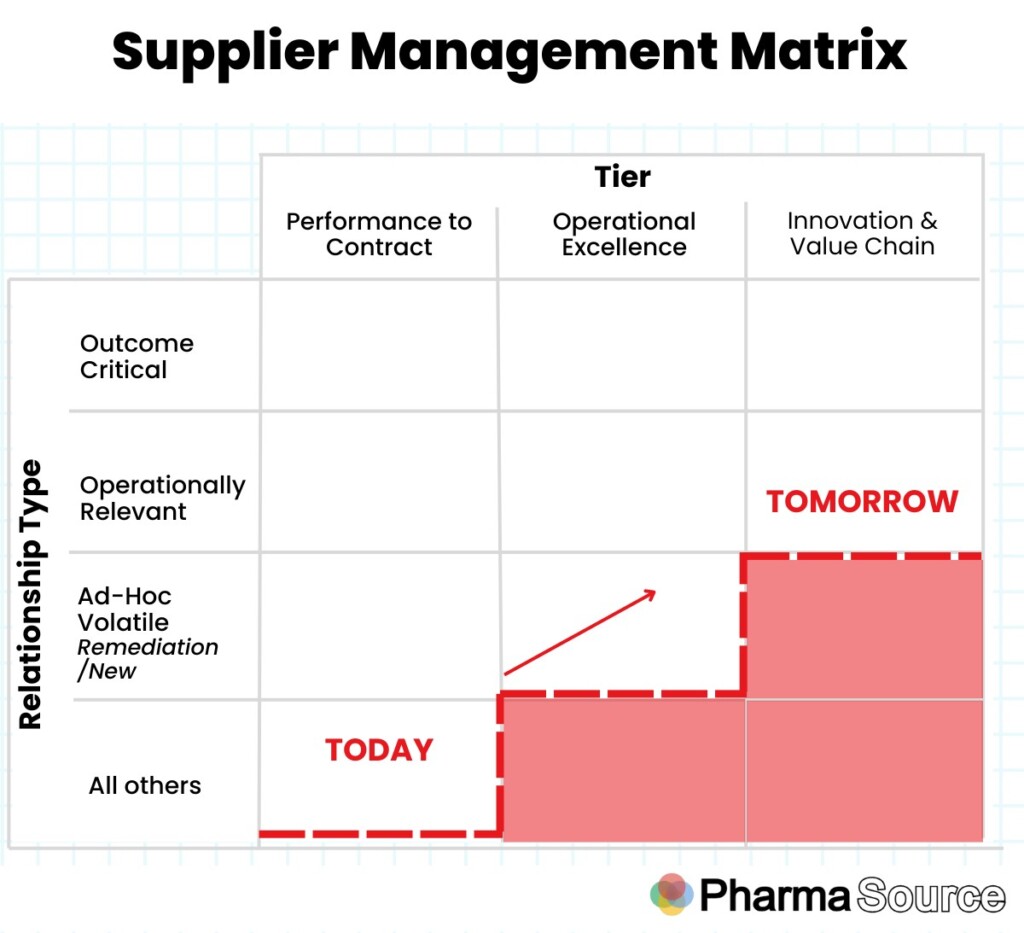
“Not every supplier can address all your business requirements from day one.”
“It’s crucial to identify which suppliers are truly strategic and where you should allocate your time and effort. To be a truly strategic partner, a supplier should significantly impact the Profit and Loss (P&L) and drive top-line growth. “
“For instance, in the R&D clinical pipeline, not all assets will directly impact the P&L over the next five or ten years.” he says.
Applying SRM principles to managing Contract Research Organisations (CROs)
Anirban highlights three key areas for applying supplier relationship management principles to contract research organisations:
1. Understanding Study Teams and Peripheral Vision
“As the R&D pipeline expands, study teams become increasingly specialised. Their primary focus is on meeting specific deliverables within tight timelines. However, they often lack a broader understanding of what other teams are experiencing.”
“When managing suppliers at the portfolio level, your role is to grasp the various dynamics within that portfolio and intervene strategically to achieve desired outcomes.”
2. Managing Variability and Risk
“Take a portfolio-wide view and identify areas where variability arises due to the proliferation of the pipeline. Acquisitions may introduce additional CROs and practices. Each deviation from standard practices carries inherent risk and cost implications.”
“Balancing autonomy for study teams with risk management becomes critical.”
3. Becoming the Expert: Know Your CRO Inside Out
Anirban emphasizes the critical role of becoming an expert on the key Contract Research Organisation to your business. “Understand their operations, identify key players, and invest in relationships. Having a direct line of communication with someone on the supplier side can significantly mitigate risks.”












 Stay ahead of trends and best practices
Stay ahead of trends and best practices
