The Pharmaceutical Supply Chain Risk Assessment Act is the latest legislation introduced in the US senate to reduce the United States’ dependence on importing drugs from foreign countries.
The legislation will have government agencies investigate supply chain weaknesses, siting the following reasons:
- 80 percent of manufacturing facilities producing active pharmaceutical ingredients are located outside of the United States.
- Overreliance on foreign manufacturing poses a national security risk in the event of a crisis, especially because more than 15 critical drug products have been in shortage for over a decade.
In a bi-partisan press release Sentors Ernst and Peters said:
“The United States cannot continue to rely on our foreign adversaries, like China, for critically important materials to meet the medical needs of Americans. I’m sounding the alarm on our compromised medical supply chain. It’s past time to reduce our reliance on bad actors and protect the health of our citizens now and in the future.” Senator Ernst said.
“Our federal government’s lack of visibility into the entire supply chain for critical medications limits our ability to address drug shortages that pose a serious national security risk and could compromise medical care for people all across the country, including service members,” Senator Peters said.
This is the latest move towards reshoring drug supplies back to the United States since 2021, when the Biden Administration first identified pharmaceuticals as a supply chain of critical importance.
Currently, the FDA lists more than 200 drugs in short supply on its online drug shortage database.
Last week the French President Emmanuel Macron announced similar plans to reshore manufacturing of 50 key medicines back to France.
A balanced relationship?
While trade of pharmaceuticals between the US and China has increased over recent years, it does not have a stranglehold on the US medicine supply chain.
As Niels Graham points out in a recent article for the Atlantic Council, trade is currently broadly balanced —the US imported $10.2B while exporting $9.3B to China—and is driven by advanced medicines such as cancer treatments and antibiotics.
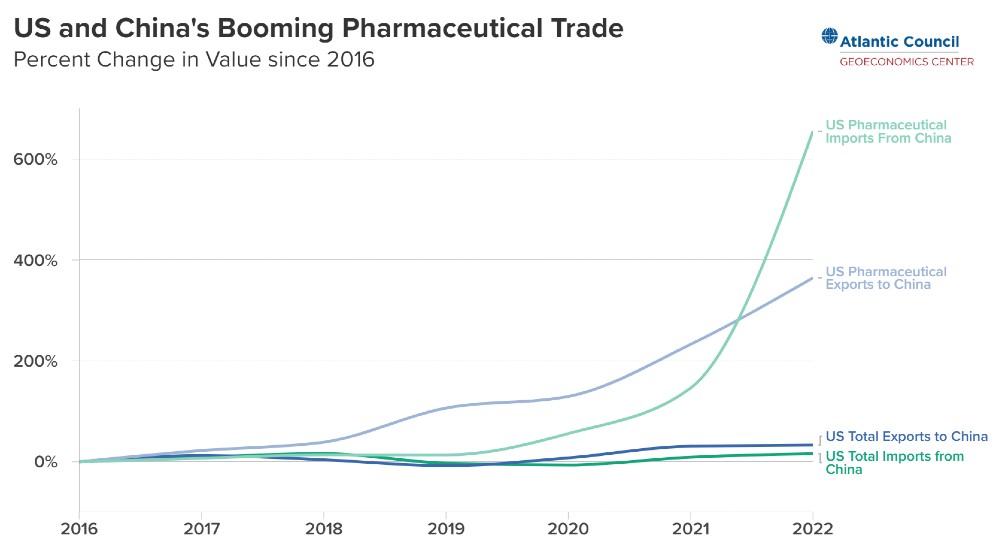
Over the past five years, two-way trade of pharmaceuticals has grown from composing just 0.6 percent of the trading relationship to nearly 3 percent of its total value.
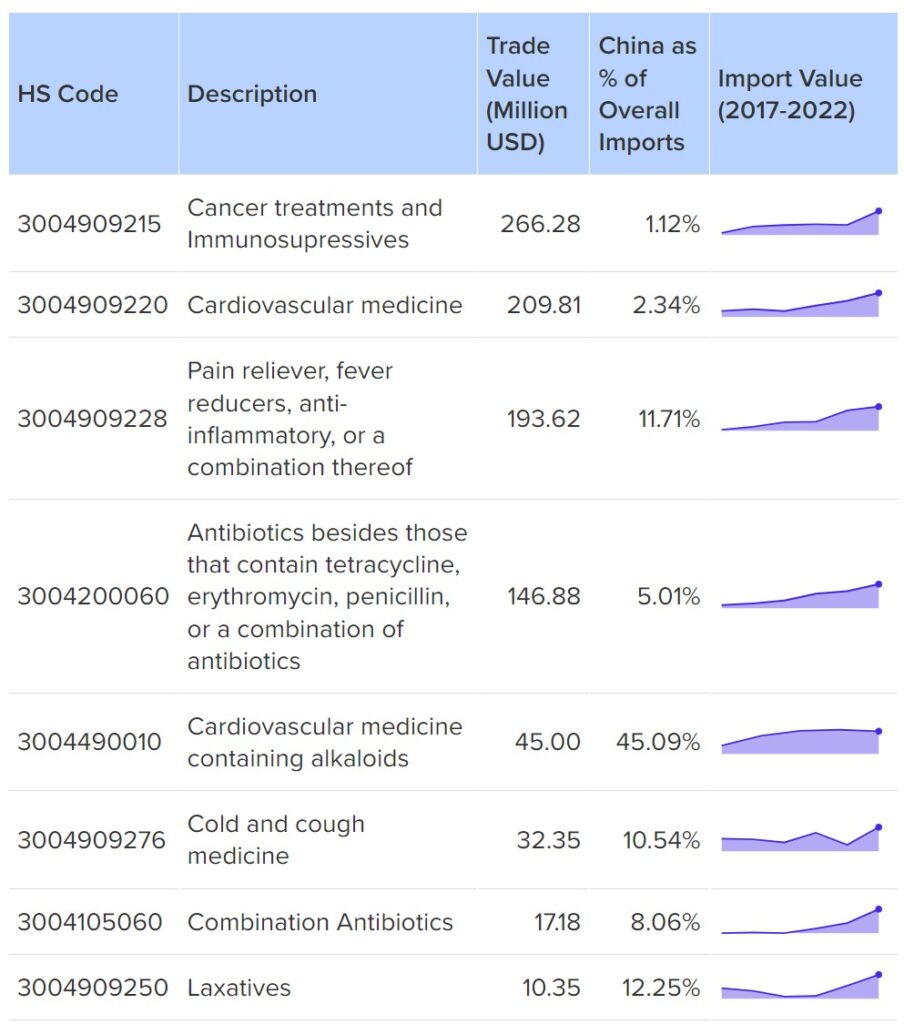
Some key drugs the US imports from China include:
Regulatory pressure to reshore manufacturing will continue to drive procurement and supply chain strategies for the foreseeable future.