The Science of CMO Selection [Video]
A framework for identifying the right contract manufacturer (CMO or CDMO) for your requirements, first time.
The selection of the appropriate contract manufacturing organization is a crucial milestone for any company and could be critical for a start-up company or for a specific program.
In this PharmaSource workshop, David Caron SVP CMC at Ayala Pharmaceuticals presented a framework of parameters and acceptance criteria to ensure a non-biased decision towards the selection of its future partner.
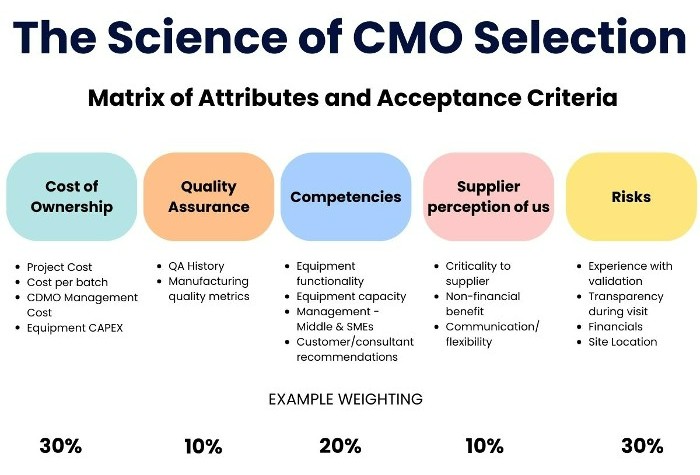
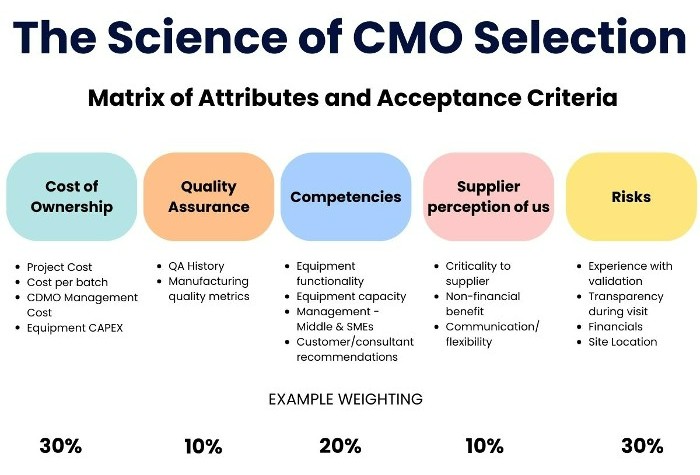
The image illustrates the matrix of attributes and acceptance criteria he explains during the talk:
Watch the full video below to learn:
- What are the most important issues for selecting a CMO partner
- How to make non-biased decisions in selecting a future partner
- How to manage Technical Transfer once a compliant material is produced
About the speaker
David Caron has more than 30 years of experience in the pharmaceutical industry developing generic and innovative drug substances and drug products. He has a wide knowledge in formulation, scale-up, technical transfer and commercialization of multiple dosage forms including pellet, tablet, coated, tablet, hard gelatin capsule, injection, semi-solid, lyophilized and radiolabeled products.
Working for start-up or larger pharmaceutical companies in Israel and Canada, he has transferred about 50 products (drug substances and drug products) to numerous contract manufacturing sites in Europe and North America and have been exposed to multicultural and challenging technical transfers.
During the set-up of new pharmaceutical companies, he took the lead to develop the quality management system and has been involved with several audits or due diligences, as well as prepared the CMC dossier for regulatory submissions (IND, NDA, IMPD, etc) and participated to pre- approval inspections adding to the chemistry manufacturing control knowledge relevant quality and regulatory experience.
