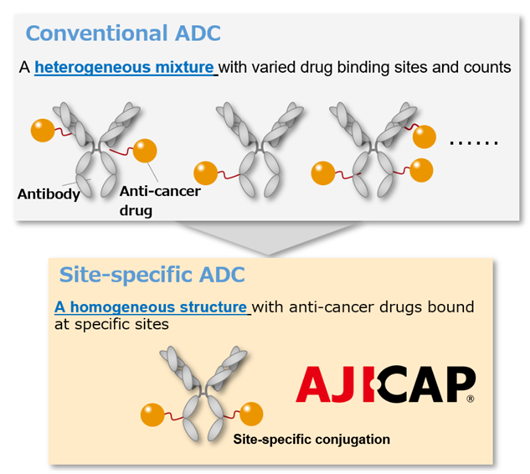
Description
Mikart, LLC is a contract development and manufacturing organisation (CDMO) with over fifty years of experience. It provides end-to-end services from early formulation development through full commercialisation. Its focus is on non-sterile oral solid and liquid dosage forms. Mikart emphasises flexibility, transparency and reliability in its partnerships with clients. It is capable of working with a diverse range of dosage forms, age populations (including paediatric and geriatric), and controlled substances (CI-CV). The organisation also supports regulatory compliance, analytical development, and clinical trial supply.
Key Products and Services
Pharmaceutical Development
• Pre-formulation studies
• Formulation development (including for paediatric and geriatric dosage forms)
• Analytical method development
Manufacturing
• Oral solid dosage forms (tablets, capsules, powders in bottles)
• Oral liquid and suspension dosage forms
• Handling of controlled substances (CI-CV)
• Clinical trial supply manufacturing
Packaging Services
• Blister packaging
• Pouch/foil strip packs
• Cartoning and tray packaging
• Unit-dose cups
• Bottle filling and sachet-type flexible packaging
Regulatory & Support Services
• Regulatory support
• Small-scale non-GMP laboratory work
• Analytical services
Mikart stands as a full-spectrum partner for pharmaceutical companies seeking to develop, manufacture, package and bring to market non-sterile oral products. With decades of CDMO experience, facilities that can scale from lab to commercial, and capabilities across regulatory, analytical, and packaging domains, the company is well-positioned to support clients looking for robust execution in complex development programmes.
Capabilities
- Analytical Methods Development
- Analytical Services
- Blending
- Blister
- Capsule
- Chemistry (CMC)
- Clinical Trial Materials
- Cold Form
- Contract Development and Manufacturing (CDMO)
- Contract Packaging
- Controlled Release
- Controlled Substances
- Dosage Form Development
- Dose Form
- Drug Product Formulation (FDF)
- Encapsulation
- Ethical
- Feasibility Studies
- Filling
- Fluid Bed Processing
- Formulation Development
- Generics
- Glass
- Granulation
- Labelling
- Liquids
- Mixing & Blending
- Non Sterile Dosage
- Oral Drug Delivery
- OTC
- Packaging
- Pouches
- Preformulation
- Process Development
- Regulatory Services
- Samples
- Serialization
- Small Molecule
- Solid Dosage
- Tablet Coating
- Tablets
- Unit Dosage