For early-stage biotechs that have reached the major milestone of obtaining capital, it is critical to transition from fundraising to drug product development as quickly as possible.
At CDMO Live 2024, Ray Sison, managing partner for SCxCMC explained how to transition from planning and raising capital to execution and operations.
In this video he discusses development budgets, hiring timing to spend, and other best practices based on his work with over 75 different clients, many of which were seed-stage or startup biotech companies.
Funding is in: Now what?
“The drug product development strategy will have a significant impact on the overall CMC budget and timing of spend. So spend a lot of time on your strategy, developing the strategy and making sure that it’s fit for your project and the commitments you’ve made to your investors.” explains Ray.
The five key steps to take are as follows:
- Review and update your living documents
- Set up / build out infrastructure
- Build / Onboard Team
- Negotiate and execute proposals
- CDMO Kickoff Meeting
Ray recommends starting with an Integrated Timeline, for the first 90 days the goal is typically to get to this red line (pictured below) is the CDMO kickoff meeting, the true transition from planning to execution.
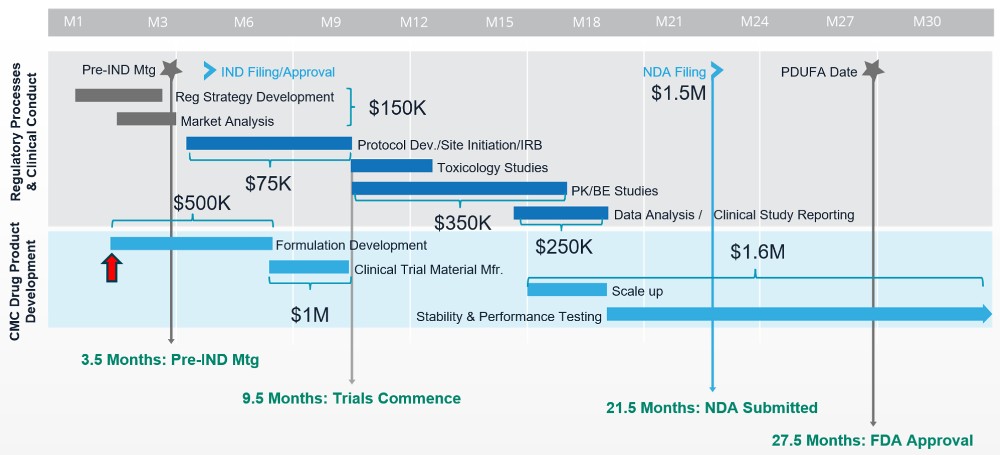
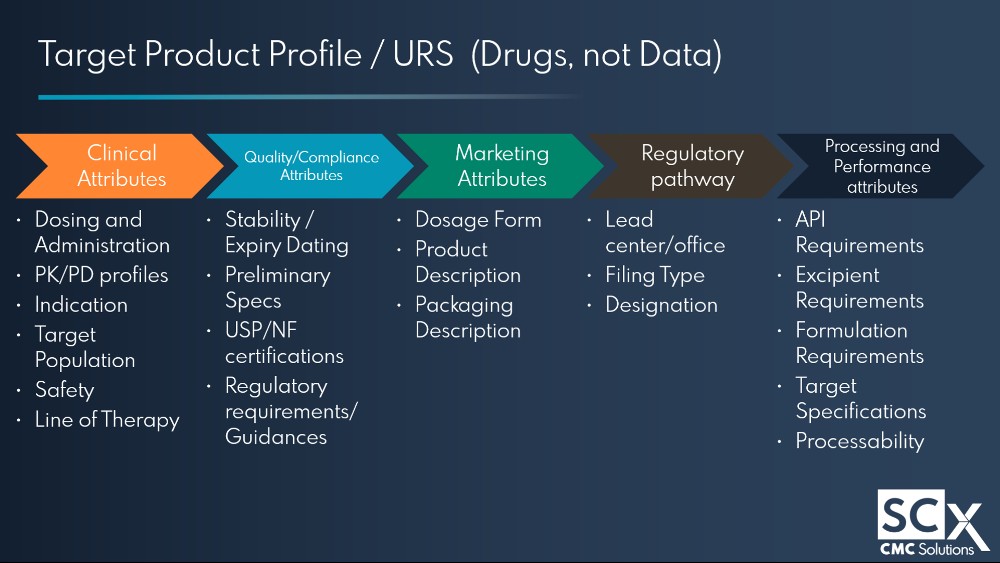
“Moving from planning stages to execution requires finalising key documents such as the Target Product Profile (TPP) and the drug product development strategy, in order to update RFPs proposals and contracts that we need to put in place with CDMOs.”
“You need to have everybody sitting at the table to have a fulsome discussion on the target product profile, and what each discipline requires from the drug product. For example, clinical quality, your marketing group, regulatory, scale up and processing and supply chain etc.”
“Working with CDMOs will require a team of talented cross functional subject-matter experts and support staff working together with the cdmo towards common goals and objectives, monitor budgets on cdmos frequently as programmes evolve and strive to provide adequate resources to manage the cdmos themselves.”
“There’s a lot of different disciplines under CMC, and so you’re going to need to build a team. The first step, know what you’re great at, know where, as an organisation, your gaps or your weaknesses lie.
Know what functionalities need to be are required to be in house, and which ones would create more value if outsourced or delegated.”
Takeaways
- Use a CDMO Kickoff Meeting as the 90 day goal for initiating CMC activities
- Revisit / Update key living documents: TPP and DP Development Strategy
- Update RFPs, Proposals and Negotiate MSAs and QTAs in a structured vendor selection process
- Assemble a balanced internal team and agree on program goals and objectives
- Monitor CDMO and Labor costs frequently
For more talks like this, watch CDMO Live 2024 On-Demand.
SCxCMC Solutions is a CMC and Supply Chain focused pharmaceutical development consulting firm specializing in guiding early to late-stage pharmaceutical/biotech firms from pre-clinical through product launch and transitioning from R&D to commercial operations.










 Stay ahead of trends and best practices
Stay ahead of trends and best practices
