Description
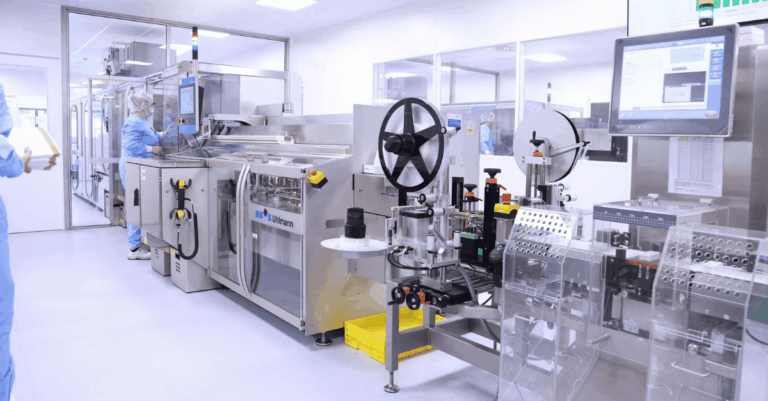
Minaris Advanced Therapies is a fully U.S.-owned global CDMO and Contract Testing Services provider formed through the combination of two highly experienced organizations. The former Minaris Regenerative Medicine, previously the cell and gene therapy division of Hitachi Chemical, brought extensive manufacturing expertise across the U.S., Europe, and Japan. It joined forces with Advanced Therapies, LLC, formerly the advanced therapies business unit of WuXi AppTec, known for its viral vector and cell therapy manufacturing, GMP analytical and biosafety testing, and analytical development services. Together, they form Minaris Advanced Therapies — a global partner with the scale, technology, and expertise to accelerate advanced therapy development and expand patient access worldwide.
Company (Headquarters):
United States
Types of Drugs Manufactured:
Cell & Gene Therapy
CDMO Services:
Drug Substance Manufacturing (API);Drug Product/FDF;Research & Development;Regulatory Services;Analytical Development;Supply Chain Logistics
Therapeutic Area:
Cardiovascular;Central Nervous System;Dermatology;Genito Urinary and Women’s Health;Hematology;Immunology;Musculoskeletal System;Oncology;Platform Technologies;Rare and Genetic Disorders;Wounds and Injuries
Facilities Location:
Europe;North America;South-East Asia
Regulatory Approvals for facilities:
USA – FDA;Europe – EMA or constituent countries;UK – MHRA;GMP;ISO 9001;Japan – MHLW
Manufacturing Technologies:
Sterile Vials
Manufacturing Information
Contact Information
Related Listings

CDMO Live Europe 2026
Experience the future of external manufacturing at CDMO Live, Rotterdam.
19-21st May 2026

External Manufacturing Leaders Boston 2026
Exclusive event for senior external manufacturing leaders. Boston, January 2026