- Medicines for Malaria Venture (MMV) and Quotient Sciences begin Phase 1 trial for long-acting injectable malaria preventive, MMV371.
- The trial, conducted in Nottingham, UK, aims to test MMV371’s safety and potential for up to three months of protection.
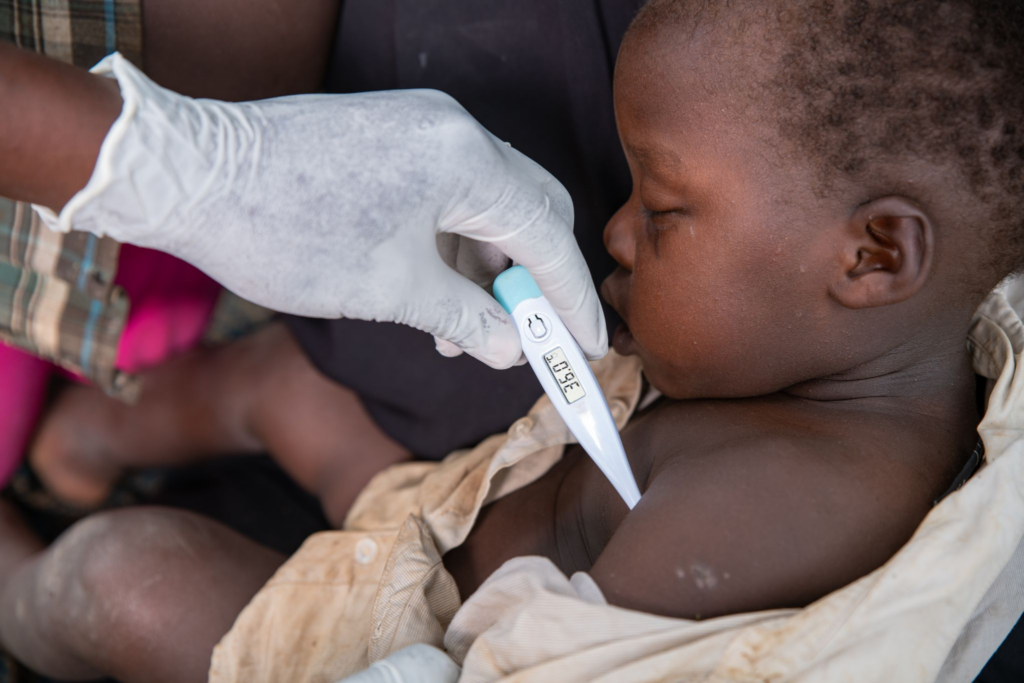
Medicines for Malaria Venture (MMV), in partnership with Quotient Sciences, has launched a Phase 1 clinical trial for MMV371, a new long-acting injectable designed to prevent malaria. The trial, currently underway with healthy volunteers in Nottingham, UK, will evaluate the safety, tolerability, and pharmacokinetics of this malaria-preventive drug.
MMV371, derived from the existing antimalarial compound atovaquone, is designed for single-dose intramuscular administration and could provide up to three months of protection. If successful, MMV371 may become an affordable and accessible addition to malaria prevention methods, particularly beneficial in areas with high rates of malaria transmission.
“This trial brings us closer to our goal of offering a long-lasting, affordable solution for malaria prevention,” said Dr Stephan Chalon, Vice President of Experimental Medicine and Clinical Pharmacology at MMV.
The drug targets both Plasmodium vivax and Plasmodium falciparum, the two most common malaria parasites, and has the potential to clear asymptomatic infections, which can contribute to the spread of malaria. The long-acting injection (LAI) formulation is intended for easy deployment in various at-risk populations, including young children, who are highly vulnerable to malaria.
Dr. Nand Singh, Medical Director at Quotient Sciences, emphasised the collaborative effort, stating, “We are pleased to support MMV with the clinical development of the antimalarial drug MMV371. The potential to help protect against P. vivax and P. falciparum strains and help save human lives is something that we are proud to be part of.”







 Overview of outsourcing trends
Overview of outsourcing trends 
