In the dynamic world of Pharma and Biopharma, the need to innovate the development and manufacturing of new drugs cost-effectively and with speed has become critically important.
As the industry continues to evolve, companies are constantly seeking ways to streamline their operations, reduce costs, and bring new innovative products to market faster. This is why Contract Development and Manufacturing Organisations (CDMOs) have grown to become a fundamental to the pharma ecosystem.
The global Contract Manufacturing market is estimated to be worth $176.5 Billion USD revenue in 2023, and forecast to reach $258.3 Billion by 2028, growing at a CAGR of 7.9% over that period.
Indeed, 90% of biopharma companies now rely on outsourcing for biopharmaceutical manufacturing, Source: BioPlan Report and Survey 2022
A CDMO can be a one-stop-shop for bio/pharmaceutical companies, providing comprehensive services from drug development to manufacturing. By outsourcing to a CDMO, pharma companies can focus on their core competencies such as drug discovery and marketing, while leaving the complex tasks of drug development and manufacturing to the experts.
- For the latest insights and best practices, make sure to register to CDMO Live – the ultimate online partnering event for bio/pharma outsourcing. Sign up here for your free ticket.
In this guide, we will delve into the world of CDMOs, exploring what they are, what they do, and why they have grown to become an integral part of the pharmaceutical industry.

- Download our CDMO European landscape infographic
What is a CDMO?
A Contract Development and Manufacturing Organisation (CDMO) is a service provider in the pharmaceutical industry that provides comprehensive services from drug development to manufacturing. In essence, a CDMO is a one-stop-shop for pharmaceutical companies, allowing them to outsource a significant portion of their operations.
The term CDMO stands for Contract Development and Manufacturing Organisation. The ‘Contract’ part of the acronym refers to the contractual agreement between the pharmaceutical company and the service provider. ‘Development and Manufacturing’ refers to the wide range of services that CDMOs provide, which can include everything from pre-formulation, formulation development, stability studies, method development, pre-clinical and Phase I clinical trial materials, late-stage clinical trial materials, formal stability, scale-up, registration batches and commercial production.
Comparison between a CDMO, a CMO, and a CRO
In the pharmaceutical industry, there are several types of service providers that companies can partner with, each offering a different range of services. Three of these are Contract Development and Manufacturing Organisations (CDMOs), Contract Manufacturing Organisations (CMOs), and Contract Research Organisations (CROs). While they may seem similar at first glance, there are key differences between them.
Contract Research Organisations (CRO) primarily focuses on providing research services. These services can include everything from early-stage research and development to clinical trials and regulatory affairs. By partnering with a CRO, pharmaceutical companies can access specialised expertise and infrastructure for their research needs.
Contract Manufacturing Organisations (CMO), on the other hand, specialises in manufacturing services. They provide the infrastructure and expertise needed to manufacture pharmaceutical products on a large scale. This allows pharmaceutical companies to bring their products to market without having to invest in their own manufacturing facilities.
Contract Development and Manufacturing Organisations (CDMO) provides a combination of both research and manufacturing services. They offer end-to-end support for pharmaceutical companies, from the early stages of research and development all the way through to manufacturing and packaging of the final product.
In essence, while CROs, CMOs, and CDMOs all play crucial roles in the pharmaceutical industry, they each offer different types of services. By understanding these differences, pharmaceutical companies can make informed decisions about which type of organisation to partner with based on their specific needs.
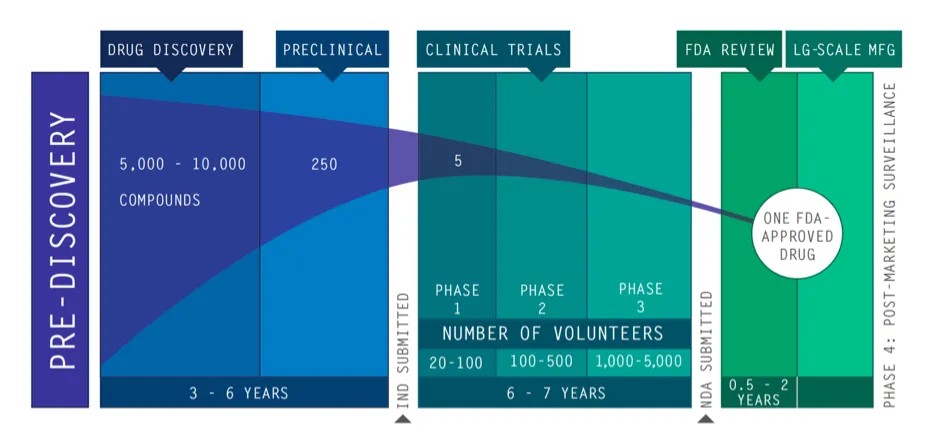
The Role of a CDMO in Drug Development and Manufacturing
A CDMO plays a crucial role in the pharmaceutical industry, providing comprehensive services that span the entire drug development and manufacturing process. This end-to-end support is what sets CDMOs apart from other service providers.
At the early stages of drug development, a CDMO can provide services such as pre-formulation and formulation development. Pre-formulation involves studying the physical and chemical properties of a drug, which is essential for designing effective and stable formulations. Formulation development then involves creating the actual drug product, which could be in various forms such as tablets, capsules, liquids, or injectables.
Once the drug formulation is developed, the CDMO can also assist with stability studies. These studies are critical for determining the shelf life of the drug product and understanding how different environmental factors might affect its stability.
As the drug moves into the clinical trial phase, a CDMO can provide clinical trial materials. These include the investigational medicinal product (IMP) that will be administered to patients during the trial, as well as the necessary documentation to ensure regulatory compliance.
Finally, once the drug is approved for marketing, a CDMO can scale up production from clinical trial quantities to commercial-scale quantities. They can also handle packaging and distribution of the final product.
In essence, a CDMO supports pharmaceutical companies at every step of the way, from drug development to commercial manufacturing. This comprehensive support allows pharmaceutical companies to focus on their core competencies, such as drug discovery and marketing, while leaving the complex tasks of drug development and manufacturing to experts.
Why Pharma Companies Use CDMOs
Pharmaceutical companies operate in a highly competitive and regulated environment. Developing a new drug is a complex, time-consuming, and expensive process. It involves numerous stages, each with its own set of challenges, from initial research and development to clinical trials and finally, manufacturing and marketing. This is where CDMOs come in.
CDMOs offer a range of benefits to pharmaceutical companies. Here are some key reasons why pharma companies choose to partner with CDMOs:
- Speed to Market: CDMOs have the expertise and resources to expedite the drug development process, helping pharmaceutical companies bring their products to market faster.
- Cost Savings: By outsourcing the drug development and manufacturing process to a CDMO, pharmaceutical companies can achieve significant cost savings. They can avoid the high capital expenditure associated with setting up their own manufacturing facilities and reduce operational costs.
- Scalability: CDMOs offer scalable solutions, allowing pharmaceutical companies to easily scale up or down their production based on demand.
- Focus on Core Competencies: By outsourcing the complex tasks of drug development and manufacturing to a CDMO, pharmaceutical companies can focus on their core competencies such as drug discovery and marketing.
- Regulatory Compliance: CDMOs are experienced in dealing with regulatory authorities around the world and can ensure that the drug development process complies with all relevant regulations.
- Access to Advanced Technologies: Many CDMOs have access to advanced technologies and specialised equipment that may not be available in-house for many pharmaceutical companies.
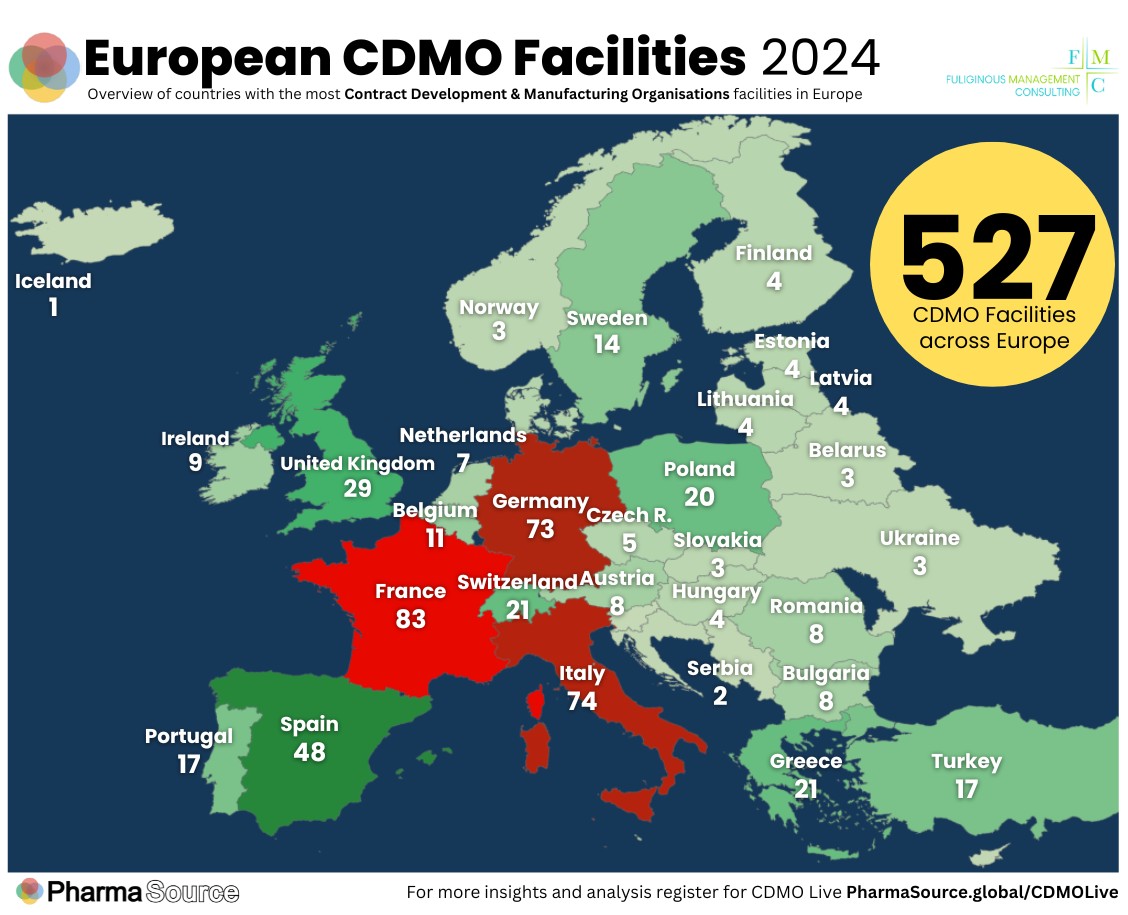
- Read our ranking of 527 CDMO manufacturing sites across Europe, which reveals the countries with the most facilities in operation.
How to choose the right CDMO for your business
Selecting the right CDMO is a critical decision that can significantly impact the success of a pharmaceutical company’s drug development and manufacturing process.
A CDMO partner will likely be a long-term strategic partnership, so it is important to make sure that they are the right fit for you company in terms of size and cultural fit.
In this video, David Caron SVP CMC at Ayala Pharmaceuticals explained his framework for selecting a Contract Manufacturing partner:
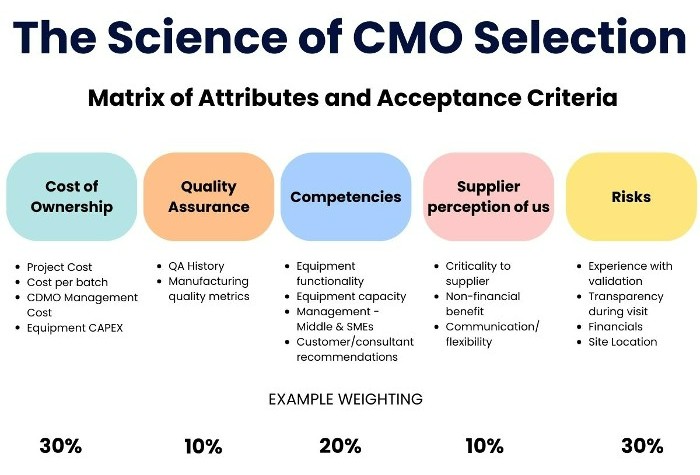
Here are some factors to consider when choosing a CDMO:
- Industry Credibility: Look for a CDMO with a strong reputation in the industry. Check their track record, client testimonials, and any industry awards or recognitions they have received.
- End-to-End Support: Ensure that the CDMO offers comprehensive services that cover all stages of drug development and manufacturing. This includes pre-formulation, formulation development, stability studies, clinical trial materials, and commercial production.
- Proactive Risk Mitigation: The CDMO should have robust risk management strategies in place to anticipate and mitigate potential challenges during the drug development and manufacturing process.
- Good Project Management: Effective project management is crucial for ensuring that projects are completed on time and within budget. Look for a CDMO with a proven track record of managing projects effectivly.
- Scalability: The CDMO should be able to scale up production quickly and efficiently to meet increasing demand.
- Trustworthiness: Trust is crucial in any partnership. The CDMO should be transparent in their operations and communication, and show a commitment to meeting their clients’ need
By considering these factors, pharmaceutical companies can choose a CDMO that not only meets their current needs but also supports their long-term goals.
The Future of CDMOs in the Pharma Industry
The pharmaceutical industry is constantly evolving, and CDMOs are no exception. As we look towards the future, several trends are likely to shape the role of CDMOs in the pharma industry.
-
- One significant trend is the increasing importance of technological capability. As drug development processes become more complex, having access to advanced technologies and specialised equipment is becoming a key differentiator for CDMOs. Pharmaceutical companies are increasingly looking for partners who can offer innovative solutions to their complex challenges.
-
- Another trend is the growing focus on specialised technologies. As pharmaceutical companies continue to explore new therapeutic areas, they require partners with specific expertise in these areas. This is leading to a rise in specialised CDMOs that offer services tailored to specific therapeutic areas or technologies.
-
- As the pharmaceutical industry continues to globalise, CDMOs with a global presence will be better positioned to serve their clients. This includes having manufacturing facilities in key markets, as well as understanding the regulatory landscape in these markets.
It’s not you, it’s me: How to part company from a CDMO
“Deciding to cut a CDMO loose may not a comfortable question asked in the presence of a CDMO”, say’s Ray Sisons, but it may be necessary.
“Working with a CDMO is a partnership but it’s also a business transaction. If one side fails to deliver, if it does not meet its contractual obligations, timeline or deliverables, then action needs to happen.”
Review contractual terms
“We use all the project management and business tools that we have at our disposal before we look at the contract and start taking legal action. If we simply view there should be an exit clause, that’s typically where you can get out for cause or without cause.”
“The red flag is if one of the sides is no longer negotiating in good faith. If that sounds like a legal term it’s because it is. It’s the language we use to mean we’ve got to the place where one or both sides become so frustrated, that they’re looking at the language of the contract to see what they’re supposed to do next.”
“In a functioning relationship, you don’t do that – you pick up the phone, you call them and you figure out a way to work it out. But if it’s evident that one of the parties is not acting in good faith, not negotiating that looking to actually solve the problem… that for me is the moment when you decide we need to look elsewhere.” says Ray.
Always have a backup
For many smaller companies having more than one CDMO working on a the same product may not be economically viable. However, there is no reason not to have backups on stand-by, says Ray.
“Always have multiples, as part of the vendor selection process. I recommend that you have a primary and you have a backup.”
“You negotiate with the primary, obviously, as you want to move forward with them. Make sure to keep a relationship with the backup, even if you don’t initially contract with them. That way if things go sideways you have options.”
Listen to the full podcast with Ray Sisons below:
CDMO Live is the ultimate event to connect the Pharma and Bio outsourcing ecosystem
Learn insights into contract manufacturing and take part in 1-to-1 partnering meetings with pre-approved Contract Development & Manufacturing Organisations.









 Overview of outsourcing trends
Overview of outsourcing trends 
