CDMO Live, is the first event of its kind to focus on partnering with the dynamic contract development and manufacturing sector.
The fast-paced four hour online event brought together expert insights around critical topics that are shaping the future of biopharma contract manufacturing.

You can now see all the content sessions from the event On Demand
From optimising contract manufacturing networks to creating business cases for biotechs, getting ahead of market shifts to de-carbonising your supply chain, here are ten strategic priorities that will be explored during the event.
- Optimise contract manufacturing networks
- Connect with innovative CDMO partners
- Learn external manufacturing best practices
- Analyse market growth trends
- Stay ahead of regulatory changes
- Reduce scope 3 emissions with partners
- Business planning for biopharma product launches
- Build better alliances
- Implement AI in GMP manufacturing
- Bridge the talent gap
1. Optimise contract manufacturing networks
At a time when all organisations are under pressure to find cost-savings while managing supply chain risks, Bayer will lift the lid on Project Martini a strategic initiative to consolidate their global network of 200 CMOs.
Malik Akhtar, VP Head of Procurement Consumer Health and David Buhmann, VP, Head of Procurement Finished Goods at Bayer will explain this critical project for their business.
The talk will be followed by a Network Optimisation roundtable when the Bayer team will share valuable insights into how they are applying network design principles to enhance efficiency, reduce costs, and ensure robust supply continuity.

2. Connect with innovative CDMO partners
Attendees will meet with a cross-section of innovative contract development and manufacturing partners who have the skills and the capacity to deliver Small Molecules, Biologics, Cell & Gene Therapy projects.
At the core of the event is our PartnerMatch matchmaking meeting programme, a curated service which connects BioPharma outsourcing leaders with relevant, innovative CDMO partners.

The platform is now open for approved buyers and CDMO sponsors to message and arrange their own meetings, so the conversations can start before and continue after the event.
- If you are already registered you can login here. Otherwise, register here
3. Learn external manufacturing best practices
We have assembled a team of experts in external manufacturing to chair a series of best practice roundtables covering key topics.
Philip Coetzee, Director of CMO Management, Daiichi Sankyo will chair a roundtable on RFP/Q Best Practices: The Right, the False and the Plausible/Questionable. This session will share valuable insights into efficient RFP/RFQ compilation, management and vendor selection strategies.

- Download our CDMO Request For Quote (RFQ) Checklist
Bernardo Estupiñán, Managing Partner at CDMO Advisor will chair a roundtable on Tech Transfer Best Practices, focusing on efficient technology transfer between organisations. Join a conversation about strategies for seamless knowledge transfer, risk mitigation, and successful project execution.
- Listen to our interview with Bernardo Estupiñán, How to Navigate Contract Manufacturing in Biotech
David Caron, SVP CMC at Immunome will share his experience in developing frameworks for CDMO selection criteria, communication strategies and risk mitigation techniques.
4. Analyse market growth trends
The biopharmaceutical landscape is forecast to change rapidly in the coming years, with biological therapeutics continuing their ascendance and healthcare systems recover from the COVID-19 pandemic.
IQVIA’s Helena Bayley will present a comprehensive overview of key trends, including growth hotspots and how clinical trial activity is shaping the pipeline of tomorrow’s medicines.
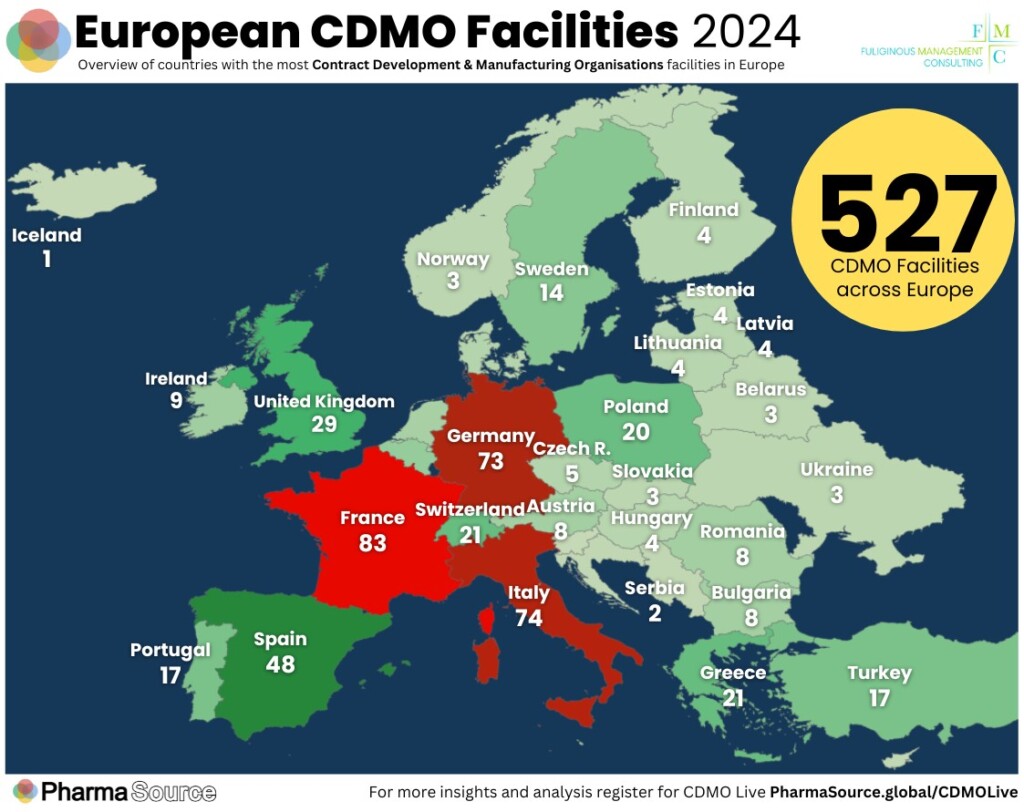
Fuliginous Management Consulting’s George Ntortas and Kyriakos Kansos will share exclusive insights based on their comprehensive research into the European contract manufacturing landscape, spotlighting key trends.
5. Stay ahead of regulatory changes
The world’s largest pharmaceutical market is at a pivotal juncture, with regulatory reforms such as the Inflation Reduction Act, BIOSECURE, and the small matter of an upcoming presidential election.
Gil Roth, President of the Pharmaceutical and Biotechnology Outsourcing Association (PBOA), will share his views on the intricate interplay between the US election outcomes, FDA and legislative dynamics.

- Listen to our podcast with Gil Roth discussing the trends shaping the CDMO Landscape in 2024
Prasfarma will be chairing an important roundtable about Annex 1 readiness, key European Union regulation from that manufacturing needs to adapt to.
Dr Jordi Botet will lead a discussion about how to align processes with the EU’s Annex 1 quality control requirements, sharing value advice on how to overcome challenges that might arise during implementation.
6. Reduce scope 3 emissions with partners
A key issue for the industry is how to collaborate with supply chain to deliver against their Net Zero and wider ESG commitments.
Rob Williams, AstraZeneca’s Director Sustainable Procurement and Vice Chair of our sustainability partner Pharmaceutical Supply Chain Initiative (PSCI) will chair an interactive roundtable about how to reduce Scope 3 emissions, sharing supplier-centric strategies for de-carbonisation.
- Read about PSCI’s latest Materiality Assessment highlighting the top supply chain issues
Dr Roxana Timmermans will present a roadmap for embedding sustainability into your Contract Development and Manufacturing processes, with actionable insights.

7. Business planning for biopharma product launches… You’ve got funding, now what?
Ray Sison will present a CMC business framework for biotech leaders for the first 90 days after funding hits your bank account.
The session will cover key issues such as how to execute a drug product development plan, which key roles you need to hire or outsource, and a roadmap for getting a new drug product into clinics.
8. Build better alliances between CDMO and sponsors
Whatever your objectives, it will only happen if you have forged strong partnerships between sponsor and CDMO, based on trust and transparency.
Kevin Wess from Grand River Aseptic Manufacturing will chair a roundtable about how to build relationships between CDMO and Sponsors. Expect to hear insights from peers into effective collaboration models, communication channels, and how to navigate challenges and resolve conflicts.

Best-selling author and founder Dr Jeff Kiplinger shares his experiences in successfully building a contract services business. His talk will focus on how win-win relationships are the key to achieving better sales and marketing success in life sciences.
- Listen to our recent interview with Jeff Kiplinger How to sell science: Advice for Better CDMO Sales and Marketing,
9. Implement AI in GMP manufacturing
Artificial Intelligence has great potential to make an impact on biopharmaceutical manufacturing – optimising processes, driving significant time savings and unlocking cost-efficiencies. However, in this highly-regulated industry, progress has often slow to make a true impact.
In the roundtable, Pragmatic AI in Pharma Manufacturing Aizon’s Claus Abildgren and David O’Gara will explain practical steps to implement AI, from ensuring compliance with Good Manufacturing Practices (GMP), to how technology can be a key enabler in unlocking powerful partnerships between CDMOs and sponsor organisations.

- Listen to our recent interview with Aizon here
10. How to bridge the talent gap
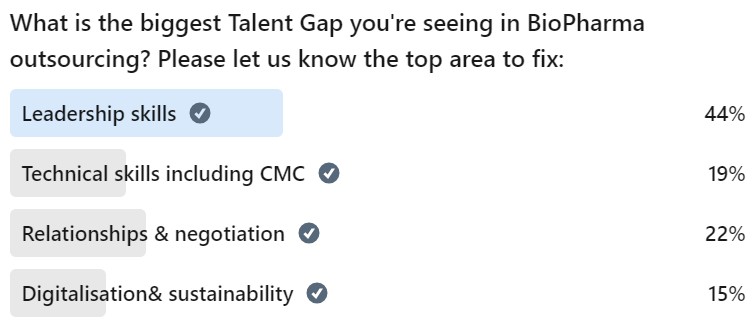
It is widely acknowledged that there is a talent shortage in outsourcing and contract manufacturing, which needs to be resolved in order for companies to meet their full potential.
Ahead of the event, we asked the PharmaSource community what they believe the greatest gap is, with Leadership emerging as the no.1 issue.
Join the Talent roundtable, hosted by Neil Kelly, CEO of Vector to discuss new approaches to talent acquisition, nurturing talent pipelines and the role of company culture in employee retention.

You can now see all the content sessions from the event On Demand










