
CDMOs are increasingly expected to provide expertise throughout the entire manufacturing process, including commercial launches.
Finding the right partner and establishing lasting relationships are essential for gaining a competitive edge, says David Blanco, PhD, PMP, Industrial BD & CDMO Director for Suanfarma.
CDMOs must offer key capabilities and global solutions that help clients achieve their goals with high-quality results at attractive costs. This requires CDMOs to focus on being innovation leaders and covering more core value areas beyond just manufacturing.
Listen to our recent podcast interview with David Blanco here:
A one-stop shop
SUANFARMA CDMO positions itself as a trusted innovative partner, providing integrated, end-to-end solutions and competitive advantages for clients. Our services cover the entire process from cell banking to drug substance release, including full regulatory support with dossier issuance to leading global regulatory agencies.
We are a true one-stop-shop partner, excelling in both supply chain and value chain solutions.
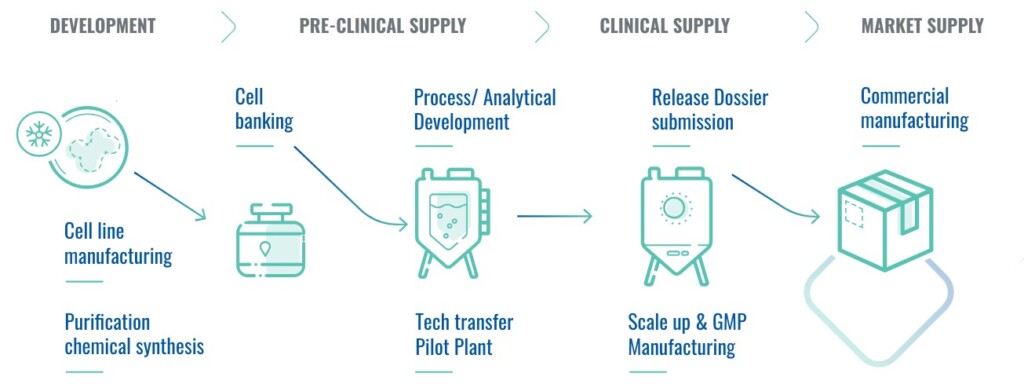
Our unique capabilities in Europe enable us to combine GMP industrial processes of fermentation, purification, and chemical synthesis within the same manufacturing plant.
This centralization simplifies the management and supply chain, leading to highly attractive costs and reduced risks through streamlined processes, operational flexibility, and time optimization.
Our strategic decisions align with sustainability goals, supported by our environmental, social, and governance (ESG) agenda. This includes full waste recycling, increased green power usage, green chemistries, and environmentally friendly processes. Our innovative strategies significantly enhance environmental sustainability, benefiting both our manufacturing efficiency and our market competitiveness.
From preclinical supply to commercial manufacturing, our services support clients through every stage of molecule development and market introduction. This is backed by our strong track record, scientific expertise, and flexible, transparent approach. CDMO manufacturers must enhance value chain performance to boost industry productivity and drive innovation. Flexibility and transparency are core values that our clients highly appreciate, allowing us to adapt to project changes, accommodate batch size requirements, and ensure timely capacity and design.
SUANFARMA CDMO has implemented policies based on PMP® procedures, offering comprehensive Project Management services, and an integrated platform for process transfer called TT&GO®. These robust methodologies and procedures minimize risks and maximize success, forming integral components of our Project Management strategy.
The solutions we offer in both the manufacturing and value chains position us as a key partner for top-tier clients. SUANFARMA is dedicated to fostering healthier lives in a sustainable world.
Let’s collaborate to bring your innovative products and therapies to success, building the core of a better life.
Meet SUANFARMA CDMO at CDMO Live, June 13th. Register for your ticket










