In the fast-paced world of drug development, time is often the most critical factor in bringing life-saving therapies to patients. Presented by BioDuro
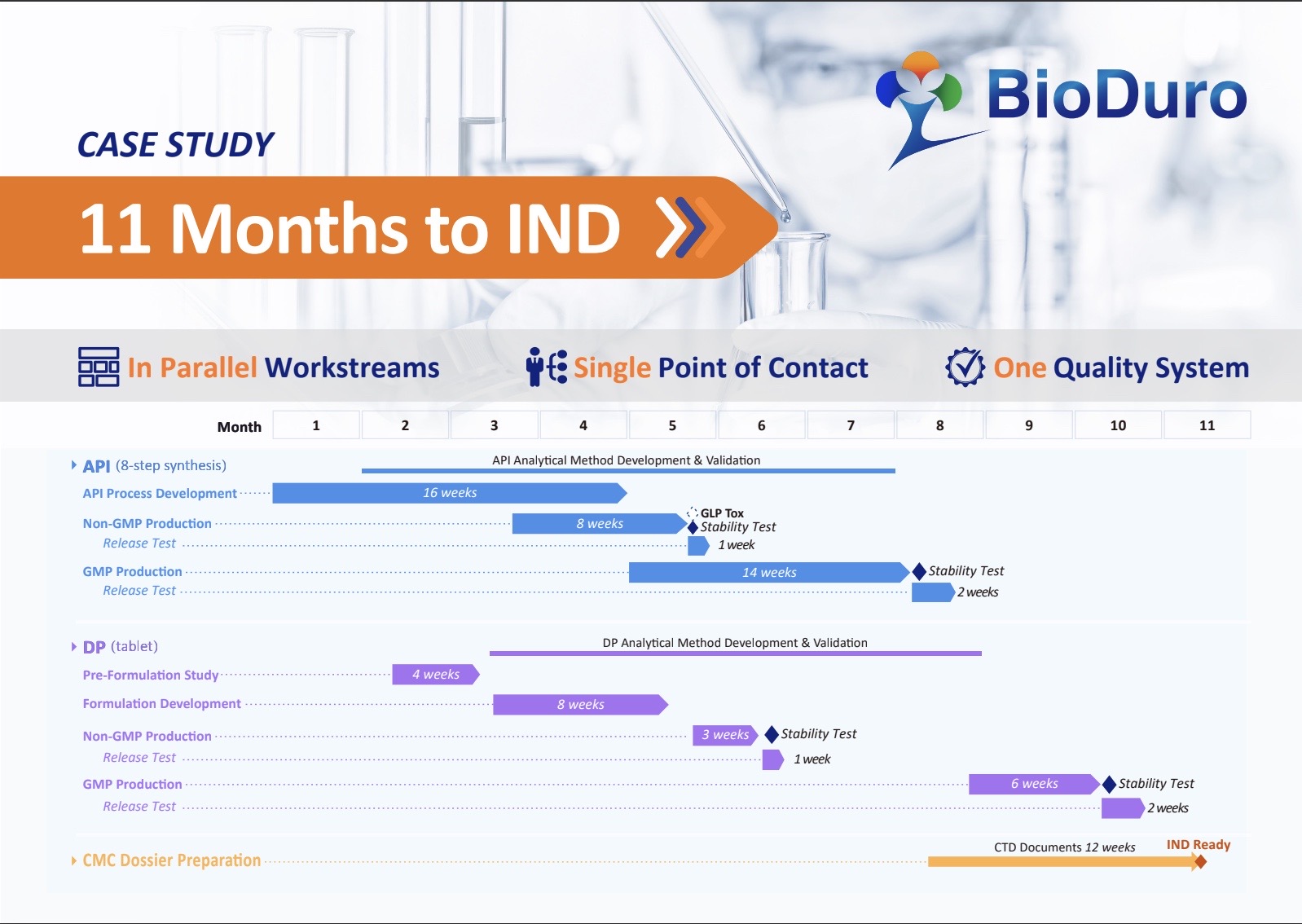
By leveraging strategic parallel workstreams, a single point of contact, and a unified quality system, we successfully accelerated the timeline for Investigational New Drug (IND) submission to the US FDA, achieving approval in just 11 months for a client’s small molecule drug project. This case study outlines the practical steps taken to accelerate development while ensuring quality and regulatory compliance.
Workstream 1: API Development (an 8-Step Synthesis)
Developing a robust and scalable API synthesis process is a critical component of any drug development program. Our approach included:
- API Process Development (16 weeks): Focused on optimizing the synthesis route, ensuring scalability, and improving yield.
- Non-GMP Production (8 weeks): Produced the initial batch for GLP-tox testing and formulation studies.
- Release Testing (1 week): Conducted comprehensive QC testing to confirm the API quality.
- GMP Production (14 weeks): Transitioned to Good Manufacturing Practice (GMP) production for GMP drug product production.
- Release Testing (2 weeks): Final verification of API quality to ensure compliance with regulatory standards.
Workstream 2: Drug Product (Tablet) Development
In parallel with API development, we advanced the formulation development and manufacturing of the drug product. Key activities included:
- Pre-Formulation Study (4 weeks): Evaluated physicochemical properties to guide formulation strategies.
- Formulation Development (8 weeks): Developed a stable, bioavailable tablet formulation.
- Non-GMP Production (3 weeks): Produced early-stage drug product materials and validated the formulation process.
- Release Testing (1 week): Conducted analytical testing to confirm consistency and stability.
- GMP Production (6 weeks): Scaled up to GMP manufacturing for first-in-human study.
- Release Testing (2 weeks): Ensured compliance with all quality requirements before proceeding to IND submission.
Workstream 3: CMC Dossier Preparation
Regulatory documentation is often a key bottleneck in the IND submission process. By initiating Chemistry, Manufacturing, and Controls (CMC) dossier preparation early and in parallel with API and DP development, we eliminated unnecessary delays.
- CTD Document Compilation (12 weeks): Developed the Common Technical Document (CTD) required for IND submission.
- IND Readiness: Ensured all necessary documentation was complete and fully compliant with regulatory guidelines, expediting the submission process.
Key Success Factors
Parallel Execution: Running multiple workstreams simultaneously, significantly reduced overall timelines with efficient material transfer and knowledge sharing
Single Point of Contact: Streamlined communication and decision-making with a dedicated project manager, preventing misalignment across teams.
Unified Quality System: Maintained consistent quality attributes across all teams
By leveraging these strategic approaches, we achieved IND submission in just 11 months—demonstrating how innovative process optimization can drive faster drug development without compromising quality or regulatory compliance. This case study provides a replicable model for other organizations aiming to accelerate their own development pipelines and bring critical therapies to patients more efficiently. For more information, please visit www.bioduro.com