Your guide to choosing the right partner for drug discovery services
In the ambitious world of pharmaceutical development, the journey from a promising molecule to a life-saving medication is filled with challenges. Choosing the right drug discovery services is a critical decision for pharma decision-makers. This in-depth guide aims to provide an insightful overview for selecting the ideal supplier, offering clarity on understanding, sourcing, and leveraging drug discovery services.
What are drug discovery services
Drug discovery is the process of identifying a compound that can be used to treat and cure diseases. A drug discovery effort typically addresses a biological target that has been shown to play a role in disease development or begins with a molecule with interesting biological activities.
Drug discovery has recently evolved significantly as a result of emerging technologies which allows the process to become more refined, accurate, and time-consuming. Drugs were discovered by identifying the active ingredient in traditional remedies or by chance, as in the case of penicillin.
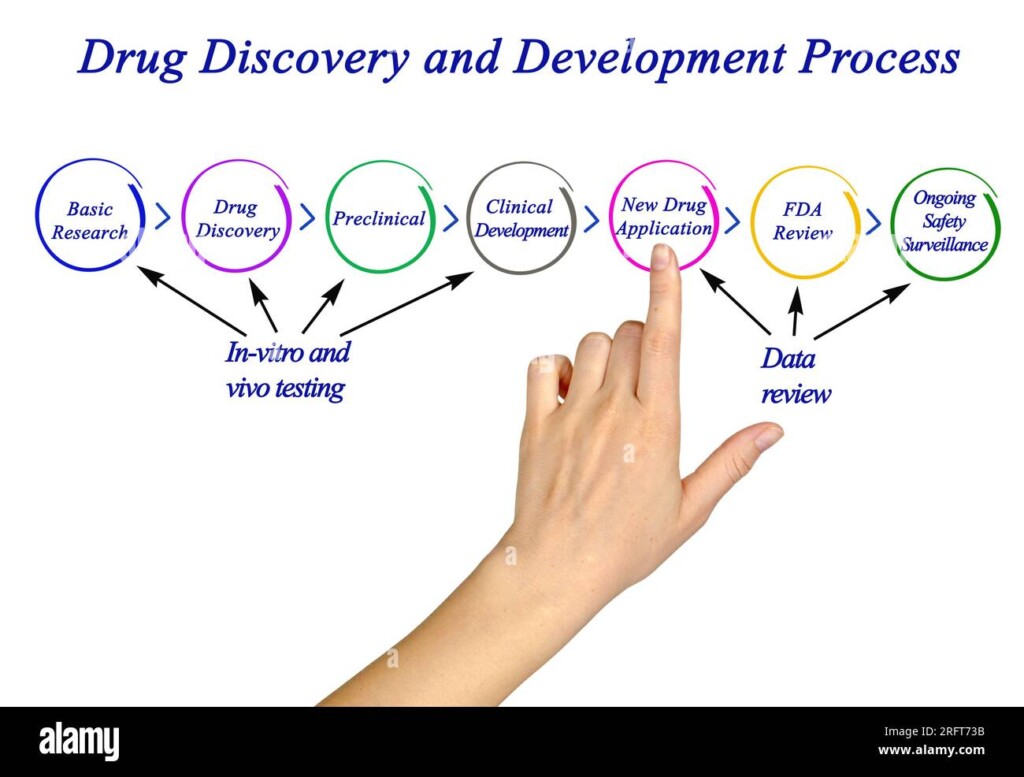
Subcategories of Drug Discovery Services
In this diverse system, many specific types are there to help with exactly what you need:
- Target Identification and Validation: Discovering possible targets for medicines and thoroughly checking if they could be effective treatments.
- Hit Identification and Lead Optimisation: Decoding nature’s secrets to find candidate molecules with the right biological activity and drug-like properties.
- Medicinal Chemistry: Chemical artistry at its finest, sculpting and refining lead molecules into potent and selective drug candidates.
- Biology Services: Unravelling the intricate dance between drugs and cells, ensuring efficacy and safety through in vitro and in vivo biological assays.
- Preclinical Development: Bringing your promising candidate to life, preparing it for the first stages of human testing.
- Computational Modelling and Simulation: Predicting drug behaviour and optimising development, utilising cutting-edge technology to accelerate lead selection and reduce risks.
- Bioanalytical Services: Quantifying drug concentrations in biological samples, ensuring accurate assessments of pharmacokinetics and pharmacodynamics.
Market trends and key drivers
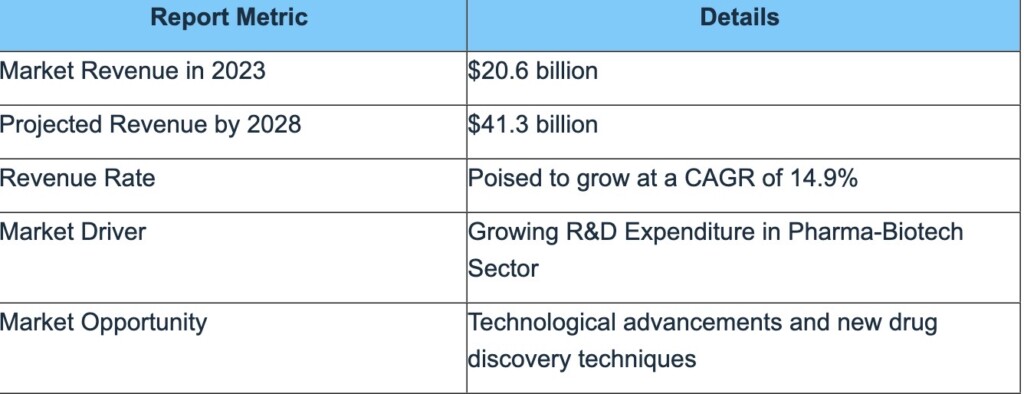
The global drug discovery services market in terms of revenue was estimated to be worth $20.6 billion in 2023 and is poised to reach $41.3 billion by 2028 growing at a CAGR of 14.9% from 2023 to 2028.
Source : Market and Markets

Key trends:
The drug discovery services market is constantly evolving, with new trends emerging that promise to revolutionise the way we find and develop new medications.
1. Artificial Intelligence (AI) Dominates the Scene:
- AI-powered drug discovery platforms: Machine learning algorithms are being used to analyze vast amounts of data, identify promising drug targets, optimise lead molecules, and predict their behaviour in the body. This accelerates the discovery process and reduces the risks involved.
- Virtual screening: AI algorithms can screen millions of molecules virtually which dramatically increases the efficiency of finding potential drug candidates compared to traditional methods.
- Predictive modelling: AI can predict how drugs will interact with cells and tissues, allowing researchers to optimise their design and avoid potential side effects.
2. Personalised Medicine Takes Centre Stage:
- Focus on individual genetic profiles and disease phenotypes: Drugs are no longer being designed for “one size fits all” solutions. Instead of that they are being tailored to individual patients based on their unique genetic makeup and disease characteristics. This leads to more effective and targeted therapies.
- Development of companion diagnostics: Alongside new drugs, diagnostic tests are being developed to identify patients who will benefit most from them. This ensures that the right drugs are given to the right people, minimising waste and maximising efficacy.
3. Decentralised Clinical Trials Gain Momentum:
- Traditional clinical trial models are being disrupted by more geographically dispersed and patient-centric approaches. This improves access to trials for patients and accelerates the drug development timeline.
- Telemedicine and digital tools are playing a key role in facilitating remote patient monitoring and data collection. This reduces the burden on patients and allows for more flexible trial designs.
4. Collaboration and Partnerships Fuel Innovation:
- Pharmaceutical companies are increasingly partnering with academic institutions, start-ups, and other drug discovery service providers. This allows them to access a wider range of expertise and technologies, accelerating innovation and reducing development costs.
- Open-source platforms and data sharing initiatives are fostering collaboration and transparency in drug discovery. This promotes shared knowledge and accelerates progress towards new treatments.
5. Focus on Sustainability and Cost-Effectiveness:
- Drug discovery services are becoming more mindful of their environmental impact, developing greener processes and reducing waste. This aligns with the growing demand for sustainable practices in the pharmaceutical industry.
- Companies are looking for ways to streamline the drug development process and reduce costs, making new medications more affordable for patients. This is particularly important in the context of rising healthcare costs.
Key drivers
The market for drug discovery services is getting bigger because of several important reasons.
- Growing R&D Expenditure: drug discovery and development have seen significant growth over the last decade in terms of clinical studies and the introduction of novel drug molecules. As per clinical trial.gov the number of registered studies went up from 32,517 in 2019 to 36,770 in 2022 at a CAGR of 4.2 %
- Accelerated Drug Development: Utilising specialized expertise and advanced technologies speeds up the pipeline, bringing drugs to market quicker and maximising return on investment.
- Focus on Core Competencies: Pharmaceutical companies can strategically delegate non-core activities like drug discovery to dedicated experts, enabling them to focus on their core strengths.
- Access to Cutting-Edge Tools: Outsourcing opens doors to advanced technologies and platforms unavailable in-house, boosting innovation potential and access to specialized equipment and knowledge.
- Globalisation of R&D: Collaborating with geographically diverse service providers unlocks a broader talent pool and expertise which further enriches the research landscape.
Benefits of Employing Drug Discovery Services
Partnering with drug discovery services brings a bunch of good things for pharmaceutical companies:
- Faster Time to Market: Get your drug to patients sooner, maximising market exclusivity and revenue potential.
- Reduced R&D Costs: Access specialized expertise and technology without the hefty investment in internal infrastructure and equipment.
- Enhanced Innovation: Tap into a wider pool of scientific talent and benefit from cutting-edge research approaches and technologies.
- Risk Mitigation: Share development risks with your service provider, minimising potential setbacks and financial losses.
- Focus on Core Competencies: Dedicate internal resources to your core strengths while leveraging external expertise for drug discovery. This approach allows you to optimise your expertise.
- Access to Advanced Technologies: Gain access to state-of-the-art equipment and platforms, ensuring your research utilises the latest scientific advancements.
Challenges
Even though outsourcing has lots of good things but we need to know there could be some tough parts too.
- Finding the Right Partner: Identifying the best fit for your specific needs, project requirements, and budget can be complex.
- Intellectual Property Concerns: Protecting your confidential drug information requires robust intellectual property agreements and vigilant oversight.
- Communication and Collaboration: Building effective communication and collaboration channels with your service provider is crucial for smooth project execution and timely updates.
- Loss of Control: Letting go of some project control can be challenging for companies accustomed to full internal development.
- Transparency and Cost Management: Ensuring cost transparency and clear communication throughout the project is essential to avoid budget overruns or unexpected charges.
Opportunities
Drug discovery services offer various opportunities for advancements in the pharmaceutical industry. Some key opportunities include:
- Innovation and Novel Therapies: Drug discovery services provide a platform for developing innovative and novel therapies. This opens doors to groundbreaking treatments for diseases and medical conditions.
- Efficiency in Research: Outsourcing drug discovery services can enhance research efficiency by tapping into specialized expertise and cutting-edge technologies. This allows pharmaceutical companies to accelerate the drug development process.
- Cost-Effective Solutions: Utilising external drug discovery services can be a cost-effective strategy. It eliminates the need for extensive in-house resources and infrastructure, allowing companies to allocate funds more efficiently.
- Access to Specialized Knowledge: Drug discovery services often involve collaboration with experts in specific therapeutic areas. This provides pharmaceutical companies with access to specialized knowledge and insights that may not be available in-house.
- Risk Mitigation: Collaborating with experienced service providers can help mitigate risks associated with the drug development process. Specialized expertise and a proven track record contribute to more reliable outcomes.
Key players
Some of the major players operating in the drug discovery services market are:
- General Electric (U.S.)
- Eurofins Scientific (U.S.)
- PPD Inc. (U.S.)
- Syngene International Limited (India)
- Wuxi AppTec (China)
- Frontage Labs (U.S.)
- Galapagos NV (Belgium)
- Aurigene Discovery Technologies (India)
- Genscript (U.S.)
- Domainex (U.K.)
- WIL Research Laboratories LLC (U.S.)
- Shanghai Medicilon, Inc. (China)
- Labcorp Drug Development (U.S.)
- Jubilant Biosys Ltd. (India)
- Evotec (Germany)
- Shanghai ChemPartner (China)
- Charles River Laboratories (U.S.)
- Merck & Co. Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
How to find the right drug discovery services partner
Finding the right drug discovery services partner is crucial for the success of pharmaceutical projects. Here are steps and considerations to help you identify the ideal partner:
Define Your Needs
- Clearly outline your project goals, timelines, and specific requirements.
- Identify the areas where you need expertise and support within the drug discovery process.
Evaluate Expertise
- Look for partners with expertise in the specific therapeutic area or technology relevant to your project.
- Assess their track record and experience in successfully delivering similar projects.
Check References
- Obtain references from previous clients or collaborators.
- Validate the partner’s reputation, reliability, and the quality of their deliverables.
Regulatory Compliance
- Ensure the potential partner adheres to regulatory standards relevant to your project.
- Check for certifications and compliance with Good Laboratory Practice (GLP) or Good Manufacturing Practice (GMP), depending on your needs.
Communication and Collaboration
- Evaluate the partner’s communication style and willingness to collaborate.
- Effective and transparent communication is crucial for successful collaboration
Infrastructure and Resources
- Assess the partner’s facilities, equipment, and technological capabilities.
- Ensure they have the necessary resources to meet the requirements of your project.
Cost Transparency
- Clearly understand the cost structure and pricing model of the partner.
- Request a detailed breakdown of costs to avoid unexpected expenses.
Intellectual Property (IP) Protection
- Clarify and discuss intellectual property rights and protection measures.
- Ensure that your proprietary information and discoveries are safeguarded.
Flexibility and Scalability
- Assess the partner’s ability to adapt to changing project requirements.
- Consider whether they can scale their operations according to the evolving needs of your project.
Cultural Fit
- Evaluate the cultural fit between your organization and the potential partner.
- Shared values, work ethics, and collaboration styles contribute to a smoother partnership.
Review Contract Terms
- Carefully review and negotiate contract terms.
- Ensure that responsibilities, timelines, and deliverables are clearly outlined in the agreement.
Ask the Right Questions in an RFP
- Craft a Request for Proposal (RFP) with targeted questions to assess the partner’s capabilities and compatibility with your project.
- Include questions about their experience, team, facilities, and approach to problem-solving.








 Stay ahead of trends and best practices
Stay ahead of trends and best practices
