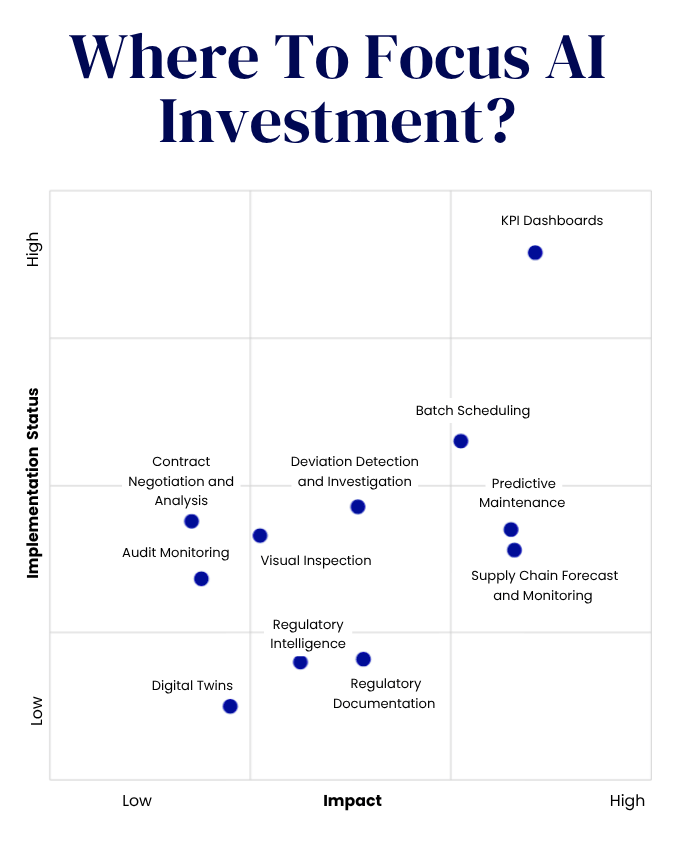
- Grifols has partnered with BARDA to test investigational ocular surface immunoglobulin (OSIG) eye drops for treating ocular damage caused by sulfur mustard exposure.
- If successful, the treatment may become one of the first FDA-approved solutions for this specific type of ocular injury.
Grifols has established a partnership with the Biomedical Advanced Research and Development Authority (BARDA) to investigate ocular surface immunoglobulin (OSIG) eye drops aimed at treating ocular damage resulting from sulfur mustard exposure. BARDA, part of the U.S. Department of Health and Human Services (HHS), is collaborating with Grifols to conduct preclinical evaluations of the therapeutic.
Sulfur mustard, also known as mustard gas, is a chemical warfare agent that can cause severe ocular injuries, including pain and photophobia. If the nonclinical studies demonstrate positive results, the U.S. Food and Drug Administration (FDA) could approve what may be one of the first treatments to mitigate the long-term effects of sulfur mustard on the eyes.
Grifols plans to repurpose an investigational OSIG therapy, currently in development for dry eye disease (DED), to target the effects of sulfur mustard exposure. The research will focus on OSIG’s anti-inflammatory properties, exploring its potential to prevent the immune system from mistakenly attacking self-antigens modified by sulfur mustard. By neutralising this immune response, OSIG may support recovery of ocular tissue.
“Grifols is applying its leadership in immunoglobulins to develop safe, effective and readily available IG-based ocular treatments,” stated Joerg Schuettrumpf, Chief Scientific Innovation Officer at Grifols. This initiative follows Grifols’ recent collaboration with Chicago-based Selagine to develop immunoglobulin eye drops for DED, which affects over 100 million individuals globally.
In addition to this partnership, Grifols’ GigaGen subsidiary has signed a contract with BARDA worth up to $135 million over six years to develop recombinant polyclonal antibody therapies for biothreats.