- SMC Pharma Services has signed a development agreement with a biopharmaceutical company to create an autoinjector platform for clinical trials in 2026.
- The Bios autoinjector platform can deliver injection volumes up to 5ml and handle high-viscosity fluids, featuring a gas-powered engine for enhanced performance.
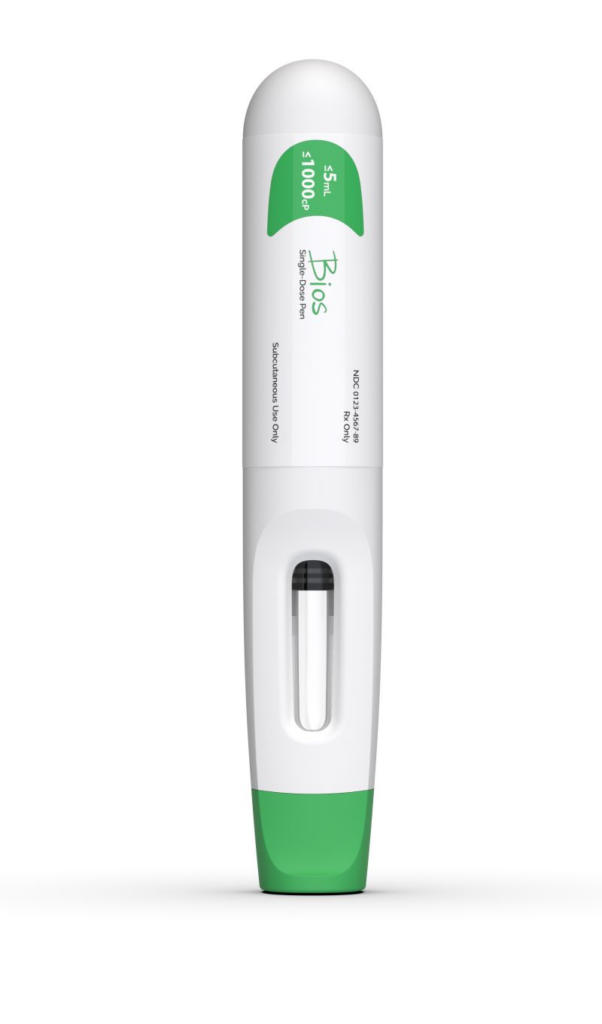
SMC Pharma Services has entered into a development agreement with a commercial-stage biopharmaceutical company focused on therapies for genetic rare diseases. This collaboration aims to develop an autoinjector platform solution for clinical trials scheduled for 2026.
The partnership will utilise SMC’s proprietary Bios autoinjector platform, which is designed to meet the market’s evolving needs. The platform accommodates injection volumes up to 5ml and is capable of handling high-viscosity fluids with viscosities up to 1000cP. Its modular design allows for flexibility and compatibility with a variety of syringe geometries, minimising the need for change parts.
A notable feature of the Bios platform is its gas-powered engine, which generates operational forces of up to 1000N. This innovation enhances the device’s power and compactness compared to traditional spring-driven systems, ensuring safe and reliable injections, even in high-stress conditions.
Uri Baruch, General Manager of SMC’s Pharma Services division, commented, “The agreement recognises the Bios platform solution built around extended functionality whilst retaining a compact and versatile format. The platform offers a great solution for biotherapies.”
With over 35 years in the healthcare industry and a global network of GMP manufacturing sites, SMC Pharma Services provides end-to-end services including drug product development and device design. Chetan N. Patel, Owner and CEO, noted that this partnership marks a significant milestone in advancing drug delivery solutions focused on patient benefits and addressing unmet therapeutic needs.















