AI is already making a real impact in drug discovery, where machine learning is increasingly valuable in driving advances in experimentation and molecule selection. However it has been slower for the pharmaceutical industry to unlock similar benefits in manufacturing.
In the latest episode of the PharmaSource podcast we spoke to Daniel O’Mahoney a Digital Transformation consultant for Körber Pharma, and lecturer in Pharma 4.0 at the National Institute for Bioprocessing Research and Training (NIBRT) in Dublin.
“The holy grail is achieving better productivity and yield.” says Daniel, explaining how it is possible for biopharma manufacturers to make a real-world impact with AI and Pharma 4.0.
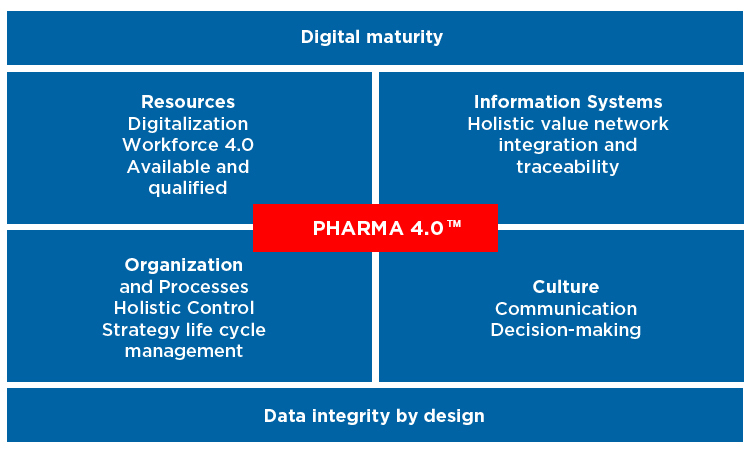
Pharma 4.0 operating model, ISPE
Challenges in biopharma
At a time when skilled workers are scarce in pharma manufacturing due to industry growth, traditional tasks such as lengthy paper forms and repetitive tests can be made easier through digital solutions. simplifying processes and enhancing job satisfaction. However, in most cases processes in manufacturing are still very manual.
There are a number of reasons why manufacturing has a long way to go with making the most of these benefits.
“There is still an awful lot to do on ‘level two’ integration of systems – it’s still a struggle to get data from one system to another” he says.
Daniel cautions that due to regulation in pharmaceutical manufacturing processes tend to be locked in. “Even if you can find a way to achieve better yield, product or a better quality improvement, sometimes you’re locked into a licence, and it’s more difficult to make a process change.”
When it comes to deciding which areas to focus your efforts on, Daniel explains that “not every technology is ready for the big time.” The first thing to look at is which other companies are having success in adopting a technology as “the second mouse gets the cheese”.
Some of the most mature and productive technologies include MES (manufacturing execution system). “If you’re paper-based site, you should move to an MES system.” says Daniel, also highlighting the maturity of electronic log books, serialisation and track and trace systems.
Start with a business case for change
The best way to get started is to define your requirements in a business case.
“Define what are the positive benefits to the business and can you put a dollar sign on it. Understand what’s in it for all the decision makers you’ll have to bring with you.” he says.







