In the latest episode of the PharmaSource CDMO Sessions, we speak Stephan Fritchi, Executive Vice President Business Units for CARBOGEN AMCIS.
Stephan is responsible for operations across all CARBOGEN AMCIS manufacturing sites worldwide, including sites in France, four in Switzerland, United Kingdom, Netherlands and China.
The contract manufacturer supports customers at all stages of drug development, from clinical trials to being released on the market as a commercial product. Their services include Highly Potent API facilities and a clean room for Antibody Drug Conjugates.
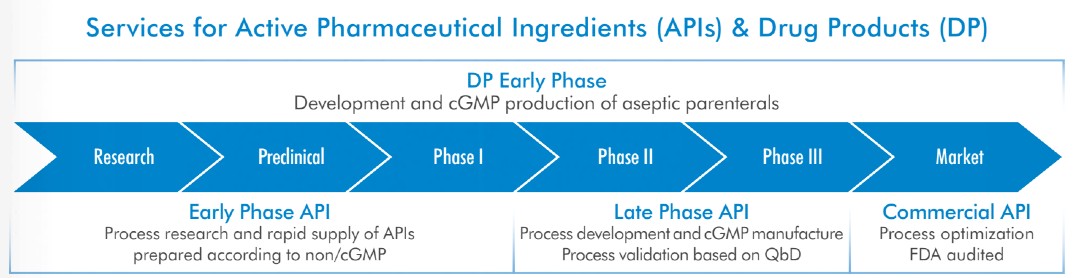
“We are specialists in providing services in developing chemical processes and produce drugs up to the final product. Depending on the nature of the projects, we can either run our activities from a single site or using multiple sites together.” says Stephan.
“We normally start our relationship with clients at a very early phase through clinical phases and then we build up a trustworthy relationship. This normally lasts five to 10 years when finally, if the product is successful on the market, we will produce commercially.”
CARBOGEN AMCIS in numbers
9
Sites in Switzerland, UK, France, Netherlands & China
20
Years experience in clinical API development
800
Employees of whom 40% hold a PhD in Chemistry
Sustainability and process optimisation
Sustainability is important for the CDMO. Their four sites in Switzerland are now proud to operate on 100% renewable electricity.
“We started this work on this years back, even before sustainability was popular in the media. Switzerland is a good place for for this endeavour, because we have a lot of hydroelectric power plants. We are also now looking to invest in more photovoltaic equipment so that we can produce our own electrical power.” says Stephan.
CARBOGEN AMCIS recently won a Chemical Industry Award for operational improvements made to their UK manufacturing site in Manchester.
“We have reviewed operational processes and looked for efficiency gains in areas such as how we handle waste material, but also where we optimised our shift working pattern to reduce cycle time and increasing efficiency and productivity by about 30%.
Best practices for partnering
Stephan shares his advice for better partnering between a sponsor and contract manufacturers.
“Over the past specially past 12-18 months inflation has become a big topic, so we need to have cost control everywhere. Customers are very sensitive towards this, which is why efficiency improvement are so important. They see that they can achieve their goal, not only cheaper because it’s delivered in a relatively short time, but it’s also quicker to get to the final product. “
The key go a good partnership is to have “a trustworthy, open and transparent relationship where you can talk about problems or challenges and solutions. If the customer is reluctant to tell us what he has already done, there is a risk that we duplicate efforts, repeating not only the good results, but also the pitfalls. The more open the communication is, the better and more efficient the collaboration will be.”
“It's important that we understand what has been done and what failed. Sometimes failure is more important than success, because from the failures you can learn.”
Stephan Fritschi
Stephan reflects that working for a CDMO that is pushing the boundaries of life sciences gives their employees a sense of purpose.
“It’s the exciting to be in the business we are in. We’re at forefront of the technology, something which is always going forward and progressing. Every individual and every employee in our organisation understands that they can make a difference with his work or her work, which makes us proud”





 Stay ahead of trends and best practices
Stay ahead of trends and best practices
