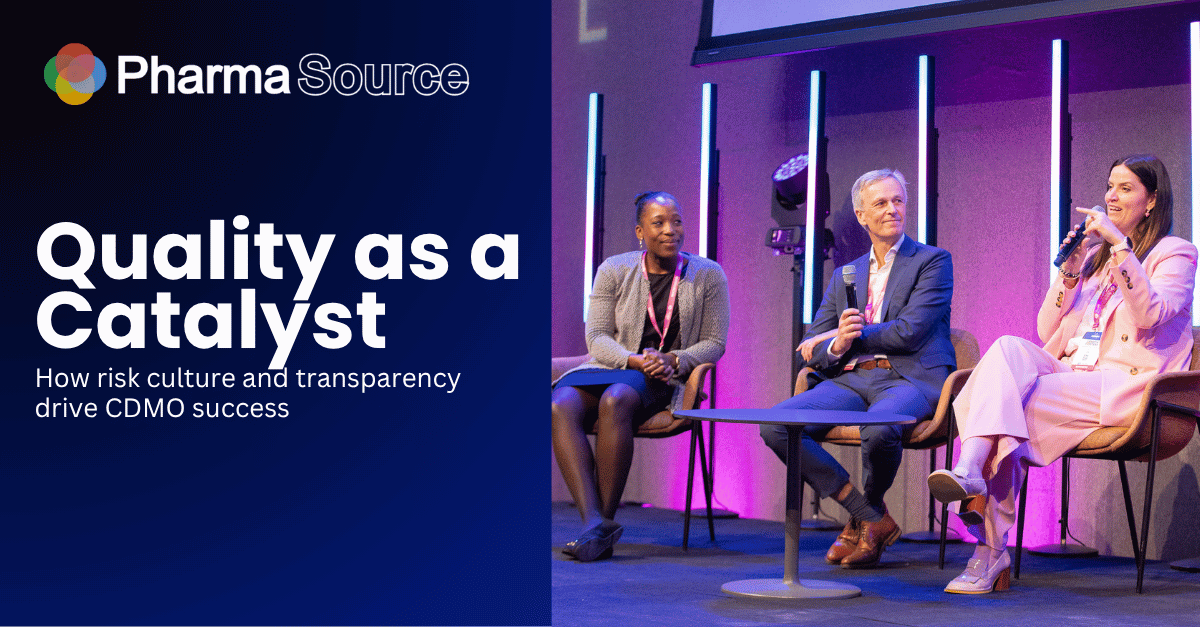
At CDMO Live 2025, a panel sponsored by Lifecore Biomedical brought together industry quality leaders to discuss how quality management can become a strategic enabler rather than a compliance burden.
The panel featured Jackie Klecker (EVP Quality & Development Services, Lifecore Biomedical), Liesbeth Foesters (VP Head of External & Clinical Supply Quality, UCB), Stefan Bouckaert (Vice President External Supply Integration Quality, Johnson & Johnson), and Gwladys Mabonzo (Associate Director External Manufacturing Vaccines, MSD).
Quality Culture Trumps Compliance
The discussion opened with a stark disconnect between sponsor expectations and CDMO priorities. Liesbeth Foesters from UCB criticised the industry’s superficial approach to quality: “While we, as an industry, are all aligned that we want the right quality product, for CDMOs it not always the first priority. Well, for us, it is.”
She emphasised that true quality goes beyond following SOPs: “I’m really talking about making sure you’re doing the right thing at the right moment. You think efficiency, but you also think business, making sure you’re doing it right is super important.”
Stefan Bouckaert from J&J reinforced this cultural emphasis: “What is for me very essential is a culture… you sense it really working with some of the companies. It can be very small CDMOs that you see have a good culture, and a willingness to really build something.”
Quality as Business Enabler
Gwladys Mabonzo presented quality as fundamental to business success: “If you want on time in full, tell me how you are going to get it. If you have batch under deviation, it’s just quality still and remain really essential, because it’s the only way to be on time.”
Foesters drew an interesting parallel: “Somebody told me that for safety, it’s very obvious, if you walk underneath a ladder, you know this could be a risk. In pharma, missing quality will come back and hit you later on. Quality is preparing for the future.”
Transparency Creates Trust
The panel unanimously identified transparency as crucial for successful partnerships. Mabonzo explained the paradox: “The more transparent you are with your customer, the less they are going to worry… The less you are transparent, the more scrutiny you are going to create. It’s just a question of fear.”
Bouckaert described how transparency affects J&J’s approach: “If there is a trust issue or there is a communication not clear, these are flagged and these are pushing the risk factor higher up… Ideal situation is we’re backing off. That’s the ideal situation.”
Critical Thinking and Soft Skills
The leaders highlighted an often-overlooked requirement: the right people with both technical knowledge and soft skills. Foesters stressed: “The profiles are very special… they need the right soft skills and capabilities to have leadership in all the conversations… They need to be able to influence.”
Bouckaert introduced the concept of “healthy paranoia” for quality professionals: “They need to be in the beginning a little bit paranoia. Hey, this could hit us if we’re not understanding.”
Measuring Success
While traditional KPIs remain important, the panel advocated for more nuanced approaches. Bouckaert revealed J&J’s sophisticated risk scoring system: “Multi factors are coming in, there’s performance metrics coming in. But even, like just, just to change guys, if you have, like, a big business coming in… we know that’s a risk factor.”
Foesters emphasised the importance of on-site presence: “We’re trying to change that and do more Gemba walks… You don’t see it in the meeting room… you see it even more when you are really going there.”
Technology: Walk Before You Run
The discussion on technology revealed practical perspectives. Mabonzo cautioned against jumping too quickly to AI: “Can we just master automation first, and then maybe we move to the next step? For me, we have so much already to build in terms of knowledge and capability when it comes to new technologies.”
Bouckaert, however, outlined J&J’s digital journey: “Actually one third of our CDMOs are already on a digital platform where we are communicating KPIs… We have 25 or so CDMOs where we have bioreactors which we are monitoring remotely.”
The session concluded with a call for quality to be repositioned from “police stopper” to strategic catalyst, requiring collaboration between CDMO and sponsor quality functions to drive this cultural transformation.
Key Takeaways
- Quality culture must permeate the entire organisation, not just quality departments
- Transparency reduces scrutiny and builds trust more effectively than hiding issues
- Quality professionals need both technical expertise and strong soft skills to influence effectively
- Risk-based approaches should guide oversight levels – less intervention is the goal
- CDMOs should proactively seek feedback and leverage customer expertise
- Digital transformation should be incremental, mastering basics before pursuing AI
- On-site presence remains crucial for understanding true quality culture