Description
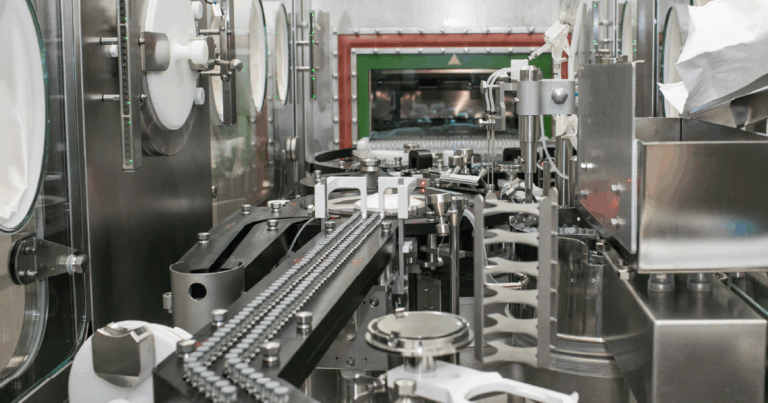
Advaxia Biologics is a specialised Contract Development and Manufacturing Organisation (CDMO) focused on advancing transformative biologics and adenovirus-based vaccines. With over 10 years of experience, the company provides end-to-end solutions for the development, manufacturing, and regulatory support of biologics, particularly adenoviral vaccines.
Based in Italy, Advaxia offers an integrated service portfolio, including expression systems, process development, analytical development, cGMP manufacturing, and quality control. They have established a successful track record, including work with major pharmaceutical companies like GlaxoSmithKline and AstraZeneca. Their state-of-the-art facilities are fully compliant with EMA, FDA, and AIFA standards.
Key Products and Services
- Adenovirus vaccine development and manufacturing
- cGMP manufacturing of clinical supply
- Expression system development
- Process and analytical development
- Quality assurance and regulatory support
- Customised CMC (Chemistry, Manufacturing, and Controls) services
Advaxia continues to be a trusted partner for pioneering pharmaceutical and biotech companies, driving innovation in the global biologics landscape.