Description
DCN Dx is a globally recognised leader in the development, manufacturing, and commercialisation of rapid diagnostic tests, specialising in point-of-use in vitro diagnostics.
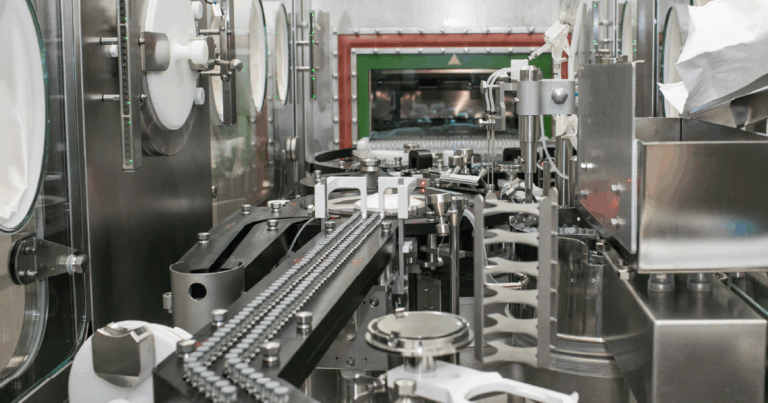
Headquartered in Carlsbad, California, DCN Dx offers comprehensive, end-to-end support for the development and production of point-of-use diagnostic devices across a diverse range of applications. Their cross-functional team of scientists and engineers collaborates to integrate all aspects of assay systems, ensuring high-performance solutions tailored to clients' diagnostic needs.
Key Products and Services
Assay Development: DCN Dx assists clients from concept to commercialisation in developing high-performance point-of-use diagnostics.
Platform Solutions: They design and deliver modern rapid point-of-use testing components, including sample collection devices, cartridges, and reader systems, ensuring maximum usability and performance.
Contract Manufacturing: Offering flexible and scalable manufacturing solutions, DCN Dx caters to production needs ranging from a few thousand to several million units.
Consulting Services: Their consulting services support the development and market introduction of innovative point-of-use diagnostic devices.
Clinical Research Services: DCN Dx provides comprehensive clinical validation and research services, developing and executing evidence plans integrated with assay design and commercialisation strategies.
Regulatory Affairs Services: Recently, DCN Dx expanded its offerings to include comprehensive regulatory affairs services, assisting clients in navigating complex regulatory landscapes.
With a commitment to innovation and quality, DCN Dx continues to be a trusted partner in the rapid diagnostics industry, providing tailored solutions that meet the evolving needs of their clients worldwide.