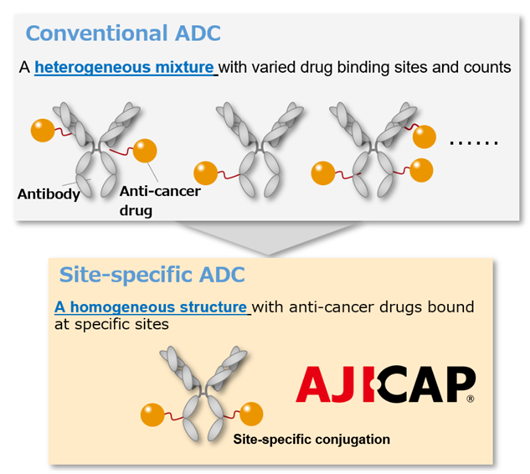
Description
GENVION Corporation is an FDA approved Contract Development and Manufacturing Organisation (CDMO) that specialises in solid dosage forms. The company is fully equipped to handle highly toxic active pharmaceutical ingredients (APIs) at Safe Bridge Level 4. With a commitment to quality and compliance, GENVION provides comprehensive contract manufacturing services that begin from the early stages of product development through to final production, ensuring reliable solutions tailored to the needs of clients in the pharmaceutical industry.
Services include:
- Contract Development and Manufacturing (CDMO)
- Contract Manufacturing (CMO)
- Manufacturing
- Encapsulation
- Filling
- Capsule
- Granulation
- Controlled Substances
- Dose Form
- Non-Sterile
- Oral Drug Delivery
- OTC
- Solid Dosage
- Spray Drying
- Tablet Coating
- Regulatory Services
- Clinical Dose
- Packaging
- Analytical Services
With its extensive capabilities and focus on safety and efficacy, GENVION stands as a vital partner for companies seeking reliable and high-quality pharmaceutical manufacturing solutions. For more information, please visit genvion.ca.