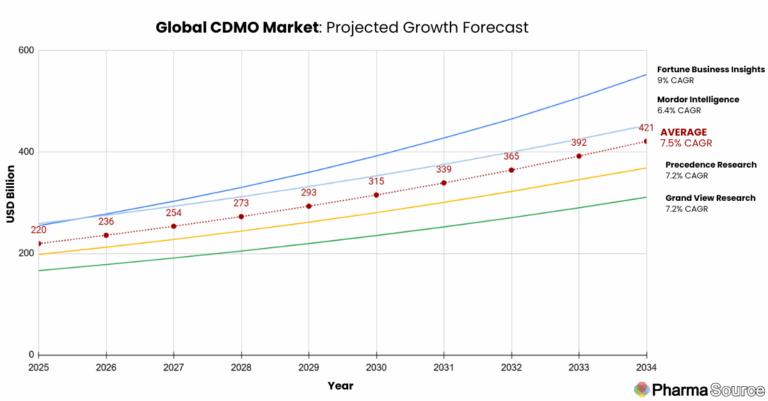
Description
Tri-Pac, Inc. is a turnkey Contract Development and Manufacturing Organisation (CDMO) that specializes in the development and filling of aerosols, liquids, foams, gels, pastes, and tubes. The company serves diverse markets including OTC, medical devices, prescription pharmaceuticals, and prestige beauty products. Tri-Pac, Inc. operates state-of-the-art facilities equipped with multiple ISO 7 and 8 cleanroom filling and blending suites designed for aerosol and liquid filling, as well as tube dispensing. The company’s capabilities are further enhanced by an on-site USP RO-EDI water system and analytical and innovation labs. Tri-Pac, Inc. has obtained numerous certifications, including ISO 13485, ISO 22716, WHO GMP, ISO 9001, ISO 14001, UL retailer certification, and Organic NOP/ANSI, and complies with regulatory standards from the US FDA, EPA, ITAR, Health Canada, and REACH.
Services include:
- 505(b)(2)
- Accelerated Stability Studies
- Aerosol
- Analytical Services
- Assay Development
- Contract Development and Manufacturing (CDMO)
- Contract Manufacturing (CMO)
- Contract Packaging
- Contract Research (CRO)
- Drug Delivery Devices
- Manufacturing
- Creams & Ointments
- Dose Form
- Non-Sterile
- Gels
- Liquids
- Nasal Delivery
- Nutraceuticals
- OTC
- Powders
- Solutions & Suspensions
- Suppositories
- Topicals
- Process Development
- Microbiological Testing
- Mixing & Blending
- Packaging
- Quality QA/QC
- R&D Services
- Serialization
- Validation
- Cleaning Equipment
- Regulatory Services
- Solid Dosage
- Non Sterile Dosage
Tri-Pac Inc remains a key player in the pharmaceutical industry, providing an extensive range of services tailored to meet various requirements in drug development and manufacturing. For more information, please visit tri-pac.us.
Capabilities
- Accelerated Stability Studies
- Aerosol
- Analytical Services
- Assay Development
- Cleaning Equipment
- Contract Development and Manufacturing (CDMO)
- Contract Packaging
- Contract Research (CRO)
- Creams & Ointments
- Dose Form
- Drug Delivery Devices
- Gels
- Liquids
- Microbiological Testing
- Mixing & Blending
- Nasal Delivery
- Non Sterile Dosage
- Nutraceuticals
- OTC
- Packaging
- Powders
- Process Development
- Quality QA/QC
- Regulatory Services
- Research & Development
- Serialization
- Solid Dosage
- Solutions & Suspensions
- Suppositories
- Topicals
- Validation