Description
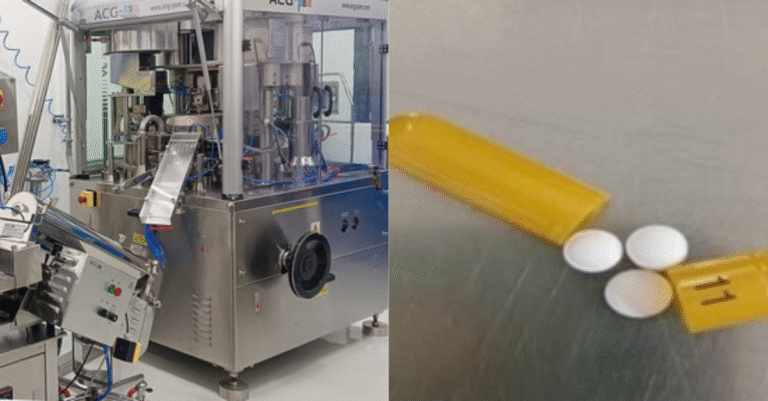
Venipharm is an independent pharmaceutical company specialising in the development and registration of generic drug marketing authorisation applications. With offices located in Paris and Hamburg, Venipharm offers cooperation opportunities to Finished Dosage Form (FDF) manufacturing partners who wish to sell their products in Europe. The company is particularly well positioned to apply for marketing authorisations and markets FDF in European markets. Additionally, Venipharm seeks FDF distribution companies to expand its export distribution channels for its own marketed products, such as Enoxaparin.
Services include:
– Finished Dosage Forms
– Marketing Authorisation Application Support
– FDF Distribution Partnerships
Venipharm is committed to enhancing the pharmaceutical industry by providing comprehensive licensing and marketing services in the European market. For additional information, please visit venipharm.com.
Capabilities
Contact Information
Related Listings

CDMO Live Europe 2026
Experience the future of external manufacturing at CDMO Live, Rotterdam.
19-21st May 2026

External Manufacturing Leaders Rotterdam 2026
Exclusive event for senior external manufacturing leaders. 26th May 2026 at CDMO Live