
Enzene has announced the launch of its EnzeneX 2.0 continuous manufacturing platform for biologics at the CPHI Milan event, which takes place from 8–10 October 2024 in Milan, Italy. The technology provides fully connected, uninterrupted processing, covering the entire production cycle from perfusion to final drug substance.
EnzeneX 2.0 leverages high titer clones and optimised cell media to improve cell productivity and streamline the production of biologics. The platform incorporates process analytical technology (PAT) to enable real-time monitoring and control, which enhances quality assurance and mitigates risks typically associated with batch-to-batch transfers.
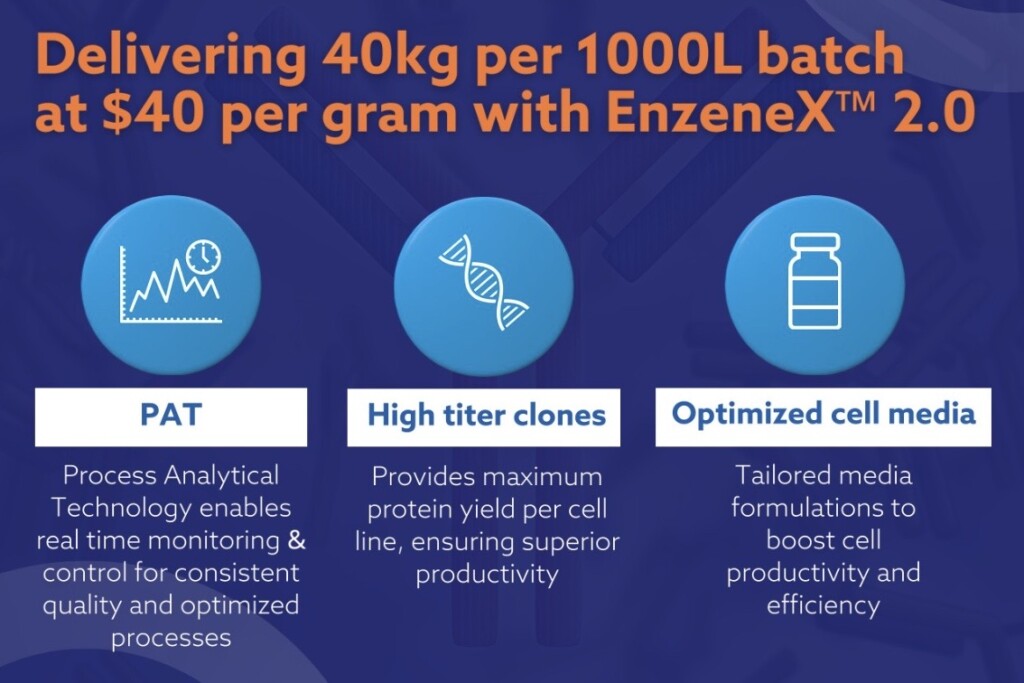
“Enzene continues to lead the industry in launching innovative approaches to maximise product yield while minimising costs,” said Himanshu Gadgil, CEO of Enzene. “With this platform, we aim to reduce production costs by up to 50%, with a targeted output of 40 kilograms per thousand-liter batch at $40 per gram.”
The system, designed to reduce equipment footprint compared to traditional fed-batch systems, supports clinical-phase good manufacturing practices (cGMP) from a 30-litre scale. Its modular design allows for flexible capacity and scalable expansion.
Additionally, Enzene is set to open a new facility in Hopewell, New Jersey, by early 2025, further supporting the company’s continuous manufacturing capabilities for its North American clients.












 Stay ahead of trends and best practices
Stay ahead of trends and best practices
