Cell and gene therapies (CGTs) represent a revolutionary approach to treating previously incurable diseases, offering new hope for patients with genetic disorders, cancer, and other life-threatening conditions.
However, bringing these complex therapies to market presents unique manufacturing challenges that differ significantly from traditional pharmaceutical production.
In this article, industry experts share their insights on overcoming the most pressing obstacles in manufacturing, from scalability issues to regulatory hurdles, providing a roadmap for advancing this promising field.
1. The Scalability Challenge
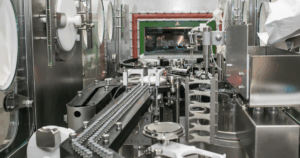
Scaling up CGT manufacturing from laboratory to commercial production presents formidable challenges unlike those seen in traditional pharmaceutical manufacturing. Unlike chemical compounds, living cells cannot simply be produced in larger batches using bigger equipment.
For autologous therapies, which use a patient’s own cells, each batch is unique and requires individual processing, making traditional economies of scale difficult to achieve. Allogeneic therapies (using donor cells) offer more scalability potential but face their own hurdles in maintaining consistency across larger production volumes. Industry leaders are pioneering innovative approaches to address these fundamental scalability obstacles:

Stella Vnook, CEO, Likarda: “Scalability remains a significant hurdle in CGT manufacturing. Autologous therapies, which are patient-specific, involve highly labour-intensive processes that are challenging to scale. To overcome this, we are investing in process automation and small-batch manufacturing systems to reduce manual intervention while maintaining product quality. Allogeneic therapies offer more scalability by using cells from a single donor to treat multiple patients. The industry has implemented master cell banks and optimized cell expansion processes to increase production efficiency. Cryopreservation also plays a key role in enabling scalability for both types of therapies, allowing for the long-term storage and global distribution of cell-based products.”
Carol Houts, Chief Strategy Officer, GermFree: “Cell and gene therapies involves complex, hands-on processes like cell isolation, expansion, and genetic modification. As demand for autologous therapies grows, manufacturers must transition from small batch, highly personalized production to scaled out decentralized manufacturing operations without compromising quality or patient safety. To address these challenges, companies are increasingly turning to automation and mobile and modular cleanrooms.”

Joel Eichmann, Co-Founder & Managing Director, Green Elephant Biotech:
“It starts with education and awareness of manufacturing in the early stages of development. While many scientists believe that scale-up and manufacturing will be solved by others at a later stage, the reality is that many processes have been transferred from academia to GMP with little change in the past.
Manufacturability needs to be considered from early stage. To do this, technology providers need to make affordable scale-down models of their manufacturing equipment available to academia and R&D.”
Veerle d’Haenens, General Manager, Global Therapy Innovations, Terumo Blood and Cell Technologies: “Scaling production for cell and gene therapies is inherently challenging due to the manual processes required, which are difficult to expand without risking product quality and consistency. These therapies often involve highly customized manufacturing steps that can vary widely, increasing the complexity of scaling from lab to clinical and commercial stages. Additionally, costs associated with scaling, from raw materials to labour, can quickly escalate, making it critical to find solutions that balance efficiency with high-quality output.
To address these issues, Terumo BCT has focused on developing automated platforms and partnering with experienced CDMOs to guide scaling efforts. These collaborations support process standardization, reducing variability and improving consistency in large-scale production.”
2. The Automation Challenge
The manual nature of CGT manufacturing creates bottlenecks, increases costs, and introduces variability that can affect product quality. Automation represents a critical solution pathway, yet implementing automated systems for biological processes that traditionally require skilled human intervention presents unique technical challenges. From cell isolation and expansion to genetic modification and quality control, each step requires specialized automation approaches. The industry is now developing novel technologies to reduce manual handling while maintaining or improving product quality and consistency:
Stella Vnook, CEO, Likarda: “Our industry has adopted automated bioreactors that precisely control cell culture conditions, reducing variability and labour costs. Additionally, we’ve integrated closed-loop processing to reduce contamination risks and enhance workflow efficiency. To maintain product quality, we use in-line sensors and AI-driven analytics that provide real-time data for quality control, allowing us to address issues proactively. These automated processes not only enhance reproducibility but also enable scalable production with greater consistency.”
Joel Eichmann, Co-Founder & Managing Director, Green Elephant Biotech: “As a technology provider, Green Elephant Biotech is addressing this challenge for the industry. We are developing tools to automate bioprocesses that are optimised for ease of use and reduction of handling time in CGT production. We use online monitoring sensors to standardise and control processes that are currently highly manual. This allows more parallel batches with less hands-on time, and more and better data.”

Augustus Kilgore, Terumo Blood and Cell Technologies: “At its core, cell therapy involves using advanced cell engineering to achieve direct therapeutic interventions, often in a very immediate timescale in the case of autologous treatments. Connecting the potential of benchtop scientific breakthroughs to the realities of broad patient population needs is where closed, automated and connected systems become crucial. With such complex operations, achieving the quality, consistency and scale needed to deliver these treatments to patients simply is not possible with open, manual processes. Automation is the critical link for realizing the full potential of this field.”
Veerle d’Haenens, General Manager, Global Therapy Innovations, Terumo Blood and Cell Technologies:“Consistent, high-quality production requires automation in both manufacturing and quality control to manage the complex production steps effectively. However, many current tools do not fully integrate with the closed, automated systems needed to maintain the high standards required for CGTs. At Terumo BCT, we have taken steps to mitigate this challenge by implementing closed-system automation, including on-line sensing technologies, to enable real-time monitoring and decision-making. Collaborations, such as with Nova Biomedical for on-line sensing, provide CGT developers with proven tools and expertise to control production quality at scale. These automated solutions reduce dependency on manual sampling, help prevent contamination, and enable more consistent production outputs, supporting the high standards necessary for CGT manufacturing.”
3. The Cost Challenge
The extraordinary cost of CGT manufacturing—with some therapies priced at hundreds of thousands or even millions of dollars per treatment—threatens to limit patient access and commercial viability. These high costs stem from complex manufacturing processes, expensive raw materials, extensive quality control requirements, and the specialized infrastructure needed for production. The unique nature of these therapies, especially patient-specific ones, makes traditional cost reduction strategies insufficient. Industry leaders are exploring innovative approaches to address this critical challenge while maintaining the highest quality standards.
Stella Vnook, CEO, Likarda: “We manage CGT costs through several key strategies. First, as an industry, we need to streamline and standardize core production steps like cell expansion and transduction, which not only reduces variability but also cuts labor and resource expenses. In the case of allogeneic therapies, we achieve economies of scale by producing large batches from single donor sources, which significantly lowers the cost per dose. We should also use predictive analytics to optimize resource use and prevent costly batch failures.”
Carol Houts, Chief Strategy Officer, GermFree: “Reducing CGT manufacturing costs requires a combination of innovation, efficiency, and strategic partnerships. Organizations like Caring Cross and Galapagos lead the way by adopting decentralized production models, minimizing logistics expenses, and leveraging automation to boost efficiency. Modular facilities and single use technologies further reduce infrastructure and operational costs. Implementing Quality by Design (QbD) principles ensures early risk management, lowering waste and rework.”
Joel Eichmann, Co-Founder & Managing Director, Green Elephant Biotech: “Green Elephant Biotech is focusing on remaining bottlenecks in the manufacturing process. For example, the expansion of adherent cells such as iPSCs or MSCs is a major cost driver due to the amount of manual work involved. Addressing these bottlenecks will enable manufacturers to reduce their cost of goods while scaling up. In addition, we’re implementing process standardisation and modular equipment to enable flexible scaling and reduced set-up costs.”

Natalia Elizalde, Chief Business Officer, VIVEbiotech: “VIVEbiotech has been working since 2015 with the main objective of developing cost-effective processes that are regulatory compliant and scalable to commercial scales. For us, cost-effective means production processes offering high titers and pure vectors able to transduce the target cell in a very efficient manner. Being fully specialized in Lentiviral vectors development and manufacture allows us to address this challenge in many different ways. One of these is by scaling up. Having many intermediate scales, from the smallest to the largest available in the market, allows us to effectively adapt to the requirements in terms of titer depending on the kind of therapy and use.”
4.The Capacity Challenge
As CGTs gain regulatory approval and patient access expands, manufacturing capacity has emerged as a significant constraint in meeting growing demand. The specialized facilities, equipment, and skilled personnel required for CGT production cannot be rapidly scaled up using conventional approaches. Limited manufacturing slots at contract development and manufacturing organizations (CDMOs) have created bottlenecks that delay clinical trials and commercial launches. Industry stakeholders are implementing creative solutions to expand capacity while navigating the unique requirements of these complex therapies.
Stella Vnook, CEO, Likarda: “To meet the growing demand for CGTs, many CDMOs are expanding their GMP-certified manufacturing facilities with modular designs that can be adapted for various therapies. We also partner with CDMOs to scale up production quickly without the need for large capital investments in new infrastructure. For allogeneic therapies, we rely on master cell banks, which provide a steady supply of starting materials that can be expanded rapidly in bioreactors, ensuring we can meet patient needs even as demand increases.”

Carol Houts, Chief Strategy Officer, GermFree: “Limited manufacturing capacity creates bottlenecks that slow the delivery of therapies, especially given the complexity of scaling individualized treatments. To overcome this, companies are investing in flexible infrastructure like modular cleanrooms, which can be quickly deployed and adapted to evolving needs. Additionally, many turn to contract development manufacturing organizations (CDMOs) to expand capacity without the high costs of building new facilities, ensuring they can meet both clinical and commercial demand efficiently.”
Joel Eichmann, Co-Founder & Managing Director, Green Elephant Biotech: “Again, automation, standardisation and simplification of manual, repetitive processes is the key to overcoming the shortage of highly skilled personnel. This can be achieved through the use of bioprocessing tools such as the platform we are developing. In addition, process intensification and the use of modular equipment in multi-use facilities can help overcome capacity issues.”
Natalia Elizalde, Chief Business Officer, VIVEbiotech: “From our point of view, current market challenges are linked more to real capabilities and expertise rather than capacity. This drove VIVEbiotech to become fully focused on Lentiviral vectors almost a decade ago, enabling us to offer the best service though our virology expertise and well-established plug and play platform. Specializing in a specific part of the manufacturing process enables a company to best channel its expertise and meet demand while not compromising quality.”
Augustus Kilgore, Terumo Blood and Cell Technologies: “There are at least two unique shifts in process that need to happen as therapies scale. The first is taking an entirely benchtop and manual process into some form of automation to meet dosing requirements for clinical trials. A fantastic solution for this transition lies within CDMOs that can truly understand the scaling process and recommend vast solutions to meet the unique needs of the cells at hand. The second shift a developer must make lies in the COGS and logistics associated with that scaling, and how to select platforms and solutions that not only meet raw material and FTE cost specifications, but also enable a simplified approach to day-to-day manufacturing.”
5. The Regulatory Challenge
The regulatory landscape for CGTs continues to evolve as agencies develop frameworks for these novel treatment modalities. Unlike conventional pharmaceuticals with well-established regulatory pathways, CGTs often require specialized approaches to safety assessment, quality control, and clinical evaluation. Navigating this complex regulatory environment while accommodating scientific advances presents significant challenges for manufacturers. The high stakes of ensuring patient safety while enabling innovation require strategic approaches to regulatory compliance.
Stella Vnook, CEO, Likarda: “Regulatory challenges are inevitable in CGT, but we take a proactive approach to minimize delays. Early engagement with regulatory bodies like the FDA and EMA allows us to anticipate requirements and avoid lengthy review processes. We also leverage expedited pathways, such as Breakthrough Therapy to speed up approvals for promising therapies. By implementing digital compliance systems, we ensure full traceability of every batch, which facilitates regulatory submissions and accelerates audits and inspections.”
Carol Houts, Chief Strategy Officer, GermFree: “The regulatory environment for CGTs presents complex challenges, particularly due to the highly personalized nature of these therapies, which makes a standardized regulatory approach difficult. Early and continuous engagement with agencies such as the FDA and EMA is essential to navigating these complexities and staying aligned with evolving requirements. The recent Regenerative Medicine Advanced Therapy (RMAT) designation granted to Kite’s Yescarta for first-line treatment in high-risk large B-cell lymphoma highlights the importance of proactive regulatory strategies.”
Joel Eichmann, Co-Founder & Managing Director, Green Elephant Biotech: “At Green Elephant Biotech, we prioritize building regulatory compliance into our process design from the outset, ensuring that tools and processes not only meet current standards but are agile enough to adapt to future requirements. This means developing technology that facilitates data compliance, process transparency, and full traceability—key elements that align with guidelines set forth by regulations such as the Biosecure Act.”
6. The Long-term Safety Challenge
Ensuring the long-term safety of CGTs represents a critical challenge due to their potential for persistent biological activity in patients. Unlike traditional medications that are metabolized and eliminated from the body, cell and gene therapies may continue to exert effects for years or even decades after administration. This creates unique requirements for safety monitoring, risk assessment, and manufacturing controls. Industry leaders are developing comprehensive approaches to address these long-term safety considerations throughout the product lifecycle, from preclinical development through post-marketing surveillance.
Stella Vnook, CEO, Likarda: “Long-term safety is critical for the success of CGTs, and we address this through rigorous testing and monitoring. The industry conducts extensive preclinical safety testing to evaluate potential off-target effects, especially in gene editing therapies. After market approval, the companies maintain robust post-market surveillance programs that monitor patient outcomes, allowing us to quickly address any emerging safety concerns. In gene therapies, the use of precise tools like CRISPR-Cas9 to minimize unintended mutations, thereby ensuring long-term efficacy and safety.”
Joel Eichmann, Co-Founder & Managing Director, Green Elephant Biotech: “Ensuring the long-term safety and efficacy of CGTs requires a robust approach to quality control and consistency in manufacturing processes. At Green Elephant Biotech, we address this by integrating rigorous online monitoring systems that enable real-time data collection, helping to track critical quality attributes consistently from batch to batch. Our emphasis on robust process design means that each step is optimised to maintain stability and reproducibility.”
7. The Collaboration Challenge
Cell and Gene development and manufacturing necessitates collaboration across a diverse ecosystem of stakeholders, including therapy developers, technology providers, academic institutions, contract manufacturers, and regulatory agencies. Effective collaboration is essential for addressing technical challenges, establishing standards, sharing best practices, and advancing the field. However, competitive pressures, intellectual property considerations, and communication barriers can impede productive partnerships. Industry leaders are pioneering new models of collaboration to accelerate innovation while protecting proprietary interests.
Stella Vnook, CEO, Likarda: “We actively engage in partnerships across the industry, working with technology providers, academic institutions, and biotech firms to bring the latest innovations into our manufacturing workflows. By participating in industry consortia, we help drive the standardization of processes and regulatory practices that benefit the entire field. Additionally, we collaborate with technology companies to co-develop next-generation bioprocessing tools, ensuring our processes remain at the forefront of innovation while being scalable and cost-effective.”
Carol Houts, Chief Strategy Officer, GermFree: “Recent collaborations are advancing CGT scalability and improving patient access. Ori Biotech and Charles River Laboratories have partnered to integrate Ori’s automated IRO platform, streamlining CAR-T production by reducing labor and costs while improving throughput and quality. Similarly, Thermo Fisher’s collaboration with UCSF aims to accelerate development and ensure faster market access for cell therapies through knowledge transfer and streamlined manufacturing processes.”

Ran Tsalic, Business Development Manager, BioProduction by SekiSui: “The cell and gene therapy (CGT) space is ever-evolving, from tweaks to current technology to completely new modalities and applications. Collaboration between therapy developers, manufacturers, suppliers, and technology companies is critical to ensure stakeholders are informed about changes in technologies, but also about manufacturability and scalability. Embracing open communication and continuous engagement with stakeholders enables BioProduction by SEKISUI to assess market demand and ultimately integrate technologies that enhance process development, manufacturing efficiency, and scalability.”
Joel Eichmann, Co-Founder & Managing Director, Green Elephant Biotech: “We’re actively collaborating with other technology providers to develop industry standards and foster a collaborative ecosystem. By working with companies across the CGT space, we focus on establishing interoperable tools and technologies that improve manufacturing efficiency and scalability. This collaborative approach allows us to pool resources and expertise to address common challenges such as automation, quality control and regulatory compliance.”
Augustus Kilgore, Terumo Blood and Cell Technologies: “We need to bring technological progress and specialized solutions out of their silos and look for better ways to collaborate that drive the industry forward, together. There has been a mindset that some single company will ultimately ‘win’ the market and that’s simply never going to be a reality. The reality is that developers will select the platforms that work best for their process, and all we can do as service and equipment providers is to demonstrate the flexibility and consistency our solutions bring to them by working together to develop data, share it openly, and be a true partner.”

Veerle d’Haenens, General Manager, Global Therapy Innovations, Terumo Blood and Cell Technologies: “Many organizations continue to rely on proprietary systems that, while potentially beneficial within a single workflow, can create obstacles for integration and compatibility across diverse platforms. In a rapidly evolving field like CGT, where innovation and adaptability are essential, isolated solutions can limit the effectiveness of therapy delivery. Collaboration that allows CGT developers to select the best tools and platforms for their unique needs, ultimately can help to speed up therapy delivery and reduce vein-to-vein time for patients. Recognizing this, Terumo BCT advocates for working together across the industry to build more effective and streamlined workflows. Our approach emphasizes developing flexible solutions and sharing data, allowing CGT developers to benefit from a range of technologies that best fit their processes.”