In the rapidly evolving field of biopharmaceutical research, Biobanks have emerged as a critical component in understanding and treating various diseases.
We recently met Dr Tony Cox OBE, Chief Executive Officer of UK Biocentre to help explain the use of biobanks, their functions, uses, challenges, opportunities, and how the pharmaceutical industry can engage with them.
What is a Biobank?
A biobank is a type of biorepository that stores biological samples, usually human, for use in research.
These samples can be blood, tissue, or other biological substances, and are often associated with detailed medical and personal data. Biobanks have become an important resource in medical research, supporting many types of contemporary research like genomics and personalised medicine
Tony explains how in biology, “most research is predicated on samples – they’re absolutely vital resources. The long term maintenance of those samples is vital for the integrity of much research that goes on.”
Biobanks are used for a variety of purposes, primarily to support research. They have become an essential tool in new drug discoveries and drug development.
“Biobanking is about how to collect, maintain, and safely store those collections of samples, so that not only are they there for reference in the future, but they can be used transactionally to support further research.”
Biobanks permit large-scale analysis for the identification of specific disease biomarkers starting from biological or digital material with well-annotated clinical and biological data. This is essential for improving personalised medical approaches, where effective biomarker identification is a critical step for disease diagnosis and prognosis.

“Anybody who’s owned a minus 80 freezer in the lab knows that keeping hold of collections and actually looking after them to a high level of quality, and maintaining and making them accessible is not straightforward.” say Tony Cox.
How Does Biobanking Work?
The biobanking process begins with informed consent from donors or research participants, ensuring ethical compliance and protecting individual rights. Samples are collected following standardized operating procedures designed to minimize variability and preserve biological integrity. Upon collection, specimens undergo initial processing, which may include separation of blood components, tissue fixation, or nucleic acid extraction.
Temperature control is paramount throughout the sample lifecycle. Blood components require preservation at -20°C for short-term storage and -80°C for long-term maintenance. Tissue samples typically require -80°C storage or cryopreservation in liquid nitrogen at -196°C to prevent degradation. Advanced biobanks employ automated storage systems with continuous temperature monitoring, backup power supplies, and alarm systems to safeguard sample viability.
Each specimen receives a unique identifier that links to associated clinical and demographic data while maintaining donor anonymity through coding systems. Laboratory Information Management Systems track sample location, processing history, quality metrics, and utilization across the biobank network. This digital infrastructure enables researchers to query biobanks for specific disease subtypes, genetic profiles, or demographic characteristics without compromising privacy protections.
Quality assurance protocols include routine audits, standard operating procedures, and compliance with regulatory frameworks such as HIPAA, GDPR, and Good Clinical Practice guidelines. Biobanks implement stringent access controls, documented chain-of-custody procedures, and ethical oversight to ensure responsible stewardship of biological materials.
Key Applications and Research Areas
Biobanks serve multiple critical functions across the pharmaceutical and biomedical research ecosystem:
Personalized Medicine Development: By analyzing genetic information stored in biobanks, researchers develop targeted therapies tailored to individual patient profiles, minimizing adverse effects and maximizing treatment efficacy. Biobanks provide the diverse patient populations necessary to identify genetic variations that influence drug response and disease susceptibility.
Disease Research and Biomarker Discovery: Access to well-characterized biological samples enables scientists to study disease progression, identify diagnostic biomarkers, and validate therapeutic targets. Population biobanks containing specimens from thousands of individuals support large-scale epidemiological studies that reveal disease risk factors and genetic predispositions.
Drug Development and Clinical Trials: Pharmaceutical companies leverage biobanks to test drug safety and effectiveness, accelerating the discovery pipeline from preclinical research through regulatory approval. Biobanked samples from clinical trial participants enable retrospective analyses, pharmacogenomic studies, and long-term safety monitoring.
Regenerative Medicine and Cell Therapy: Stem cell banks, organoid repositories, and cell line collections provide essential materials for developing next-generation therapeutics. These specialized biobanks support research in tissue engineering, disease modeling, and personalized cell-based treatments.
Precision Oncology: Cancer biobanks collect tumor tissues, blood samples, and patient-derived xenografts that enable researchers to characterize tumor heterogeneity, identify resistance mechanisms, and develop companion diagnostics for targeted therapies.
Market Overview
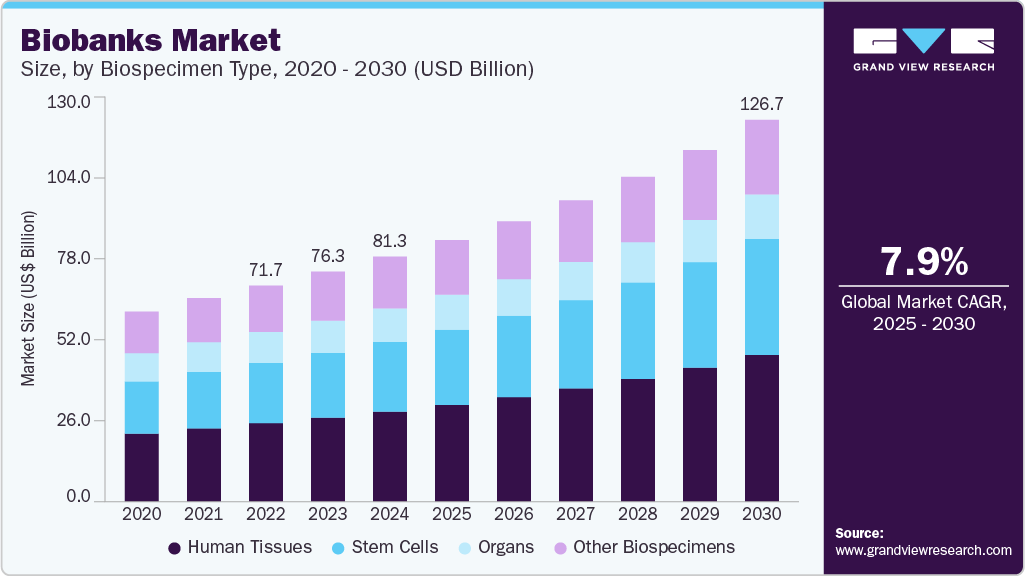
The global biobanks market size was estimated at USD 81.29 billion in 2024 and is projected to reach USD 126.69 billion by 2030, growing at a CAGR of 7.85% from 2025 to 2030. High investments in the R&D of advanced therapies, such as regenerative medicine, personalized medicine, and cancer genomic studies, are some of the driving factors.
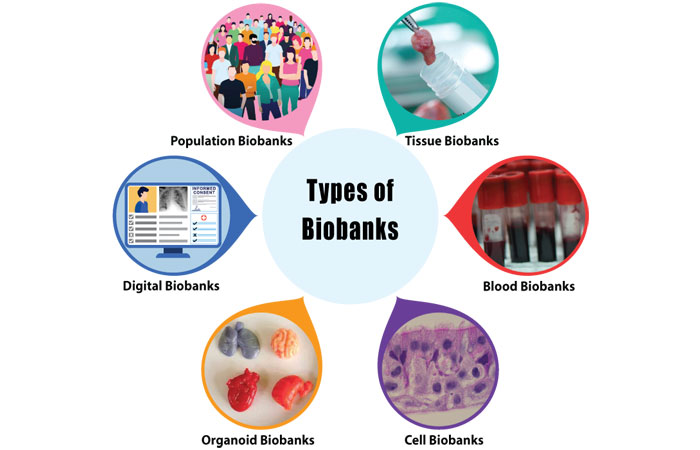
Types of Biobanks in Pharmaceutical Manufacturing
The pharmaceutical industry utilizes several specialized biobank categories:
Tissue Biobanks: Store surgical specimens and autopsy materials fixated for histological examination, requiring -80°C storage or liquid nitrogen preservation with cryoprotectants to prevent cellular damage.
Blood Biobanks: Maintain serum, plasma, and cellular components in test tubes with preservatives for biochemical and DNA analysis, preserved at -20°C short-term or -80°C for extended storage.
Cell Biobanks: Repositories like the American Type Culture Collection and Korean Cell Line Bank provide immortalized cell lines, primary cells, and stem cells for pharmaceutical research and manufacturing.
Organoid Biobanks: Emerging repositories of mini-organs cultivated from stem cells that replicate organ structure and function, enabling disease modeling and drug screening applications.
Digital Biobanks: Integrate biospecimen data with electronic health records, genomic sequences, and imaging data, creating comprehensive datasets that support artificial intelligence applications and multi-omics research.
Population Biobanks: Large-scale repositories containing thousands to millions of specimens from specific populations, enabling genome-wide association studies and population health research.
Growth Factors: What is Driving Market Expansion?
Multiple converging factors are propelling biobanking market growth:
Precision Medicine Adoption: The increasing emphasis on precision medicine and population genomics is one of the most prominent drivers reshaping the biobanking market, fueled by cutting-edge genomic technologies, enhanced data analytics, and the shift toward personalized healthcare approaches.
Cell and Gene Therapy Pipeline: Cell and gene therapies have emerged as highly targeted treatments with the potential to address medical needs that traditional drugs often cannot tackle. In 2024, 38 such treatments had already gained US FDA approval, driving demand for specialized biobanking infrastructure.
Chronic Disease Prevalence: The increasing prevalence of chronic diseases such as cancer, diabetes, cardiovascular disorders, and neurodegenerative conditions is a major driver, as these diseases require long-term research dependent on access to high-quality biological samples.
Pharmaceutical R&D Investment: High investments in R&D of advanced therapies, such as regenerative medicine, personalized medicine, and cancer genomic studies, are driving factors behind biobanking expansion.
Automation and Digital Transformation: End-to-end automation for sample handling and storage, combined with AI-powered biobank management systems, enhances operational efficiency, regulatory compliance, and accelerates biomedical research.
Regulatory Support: Strong public investment with national research networks and collaborations support disease-focused biobanking, while growing focus on quality standards, open science, and international collaboration support market growth.
Challenges for Biobanks
However, the field of biobanking does faces several challenges. The required biobanking infrastructure is currently fragmented and not prepared for the biobanking of microbiomes. The rapid advancement of technologies requires an urgent assessment of how biobanks can underpin research by preserving microbiome samples and their functional potential.
Security risks are another significant challenge for biobanks.
Regardless of the type of security used, biobanks are exposed to natural hazards (earthquakes, fires, floods), technical hazards (such as power outages) or damage caused by intrusions and theft of biological samples, computer hacking, or loss of confidential data. Along with technical issues, biobanking frequently raises important privacy and security issues that must be resolved as biobanks continue to grow in scale and scope.
Tony Cox explains that “having knowledge of a person’s DNA is not only a gross violation of privacy, but it also opens up significant vulnerabilities. I can understand why there is increasing concern and nervousness about what is happening to that data, especially as very large amounts are being collected now. It’s unclear what the long-term opportunities for bad actors to exploit this will be. Therefore, it’s crucial that we think very long and hard about how to protect that data.”
“As a biobank, we don’t collect any data about our samples that we manage, other than what’s necessarily generated as part of the processing. We don’t hold any patient-specific information. However, there is an increasing trend for both companies and research organisations to use trusted research environments to aggregate and hold their data. These environments are very secure and have strong safeguards for managing access to that data, with no direct visibility of the data.”
These developments help assure participants in these large studies that their data will be managed responsibly and securely for many years to come. It’s not only the implications for the individual that matter, but also for their descendants, their children, their grandchildren. When you begin to think through those implications, it’s a very significant subject that we must think about deeply and get right.”
How pharma professionals should engage with biobanks
Biobanks have become an essential tool for new drug discoveries and drug development. They play an important role in the whole process of patient prevention and prediction, follow-up, and therapy monitoring and optimisation. Therefore, it is crucial for the pharmaceutical industry to establish collaborations with biobanks to access valuable biological samples and associated data for their research and development activities.
Tony Cox recommends getting in touch with biobank providers at the earliest stage of a project.
“If you’re running a study or a clinical trial, thinking about the end game of that trial can be really important. Many of the resources that are collected during a trial need to be preserved potentially for years or even decades.”

“We advise on the collection strategies for those trials, but also on the long-term storage. Something as simple and mundane as choosing the right tube for samples to end up in could have cost implications. Savings in the hundreds of thousands of pounds could be realised if things are done in the right format and in the right way.”
Listen to the full interview with Dr Tony Cox.
Frequently Asked Questions (FAQ)
What is the difference between a biobank and a biorepository?
Although the terms are often used interchangeably, a biorepository typically refers to the physical facility used for storing biological samples. A biobank, however, represents a broader ecosystem that includes not only storage, but also data management systems, governance structures, ethical oversight, sample processing workflows, and controlled distribution. In pharmaceutical manufacturing, CDMOs generally operate full biobank models rather than simple storage repositories.
How long can biological samples be stored in biobanks?
The storage life of biological samples depends on the sample type, preservation method, and quality controls in place. Under validated cryogenic conditions at -80°C or in liquid nitrogen (-196°C), many samples can retain integrity for decades. DNA samples may remain viable for over 20 years, while properly cryopreserved cells and tissues can be stored indefinitely with routine quality monitoring. However, temperature excursions and repeated freeze-thaw cycles can significantly reduce sample stability.
What regulatory frameworks govern biobanking operations?
Biobanking is regulated by a combination of regional and international frameworks. In the U.S., operations must comply with FDA guidance, CLIA, HIPAA, and state-level biobanking laws. In Europe, GDPR, national legislation, and EU tissue directives apply. Biobanks supporting clinical trials and commercial manufacturing must also follow GCP and GMP standards. International guidelines such as ISO 20387 and ISBER best practices help harmonize quality across jurisdictions.
How do biobanks protect donor privacy and maintain sample anonymity?
Biobanks protect donor privacy through informed consent, coded sample identifiers, and strict access controls. Personal data is separated from sample information using encrypted linkage systems. Many research applications rely on fully de-identified or anonymized samples. Additional safeguards include role-based system access, audit trails, cybersecurity controls, and restricted physical access. CDMOs must comply with major privacy regulations such as GDPR and HIPAA, with ongoing audits and staff training.
What is the role of AI and machine learning in modern biobanking?
AI and machine learning enhance biobanking by automating inventory management, predicting sample degradation, and optimizing storage and retrieval. These tools also analyze complex genomic and clinical datasets to support biomarker discovery, patient stratification, and drug response prediction. AI is increasingly used for predictive maintenance of cryogenic equipment, logistics optimization, and even automated sample quality assessment through computer vision and digital pathology.
How do virtual biobanks differ from traditional physical biobanks?
Virtual biobanks operate as federated digital networks that connect multiple physical biobanking sites through a centralized data platform. Samples remain stored locally, but researchers can search across institutions based on clinical or molecular criteria. This model improves access to diverse sample sets, reduces duplication, and enables large-scale collaboration while allowing institutions to retain control of their collections. For pharmaceutical companies, virtual biobanks expand access to geographically and demographically diverse specimens.








