A Contract Development and Manufacturing Organization (CDMO) is a company that provides drug development and manufacturing services to the pharmaceutical industry. CDMOs play a crucial role in helping pharmaceutical companies and biotechs bring new medicines to market efficiently and cost-effectively.
A CDMO can be a one-stop-shop for biopharmaceutical companies, providing comprehensive services from drug development to manufacturing.
90% of biotechs now rely on outsourcing for manufacturing [source: BioPlan Report and Survey 2022] so they can focus on their core competencies such as drug discovery and marketing, while leaving the complex tasks of drug development and manufacturing to the experts.
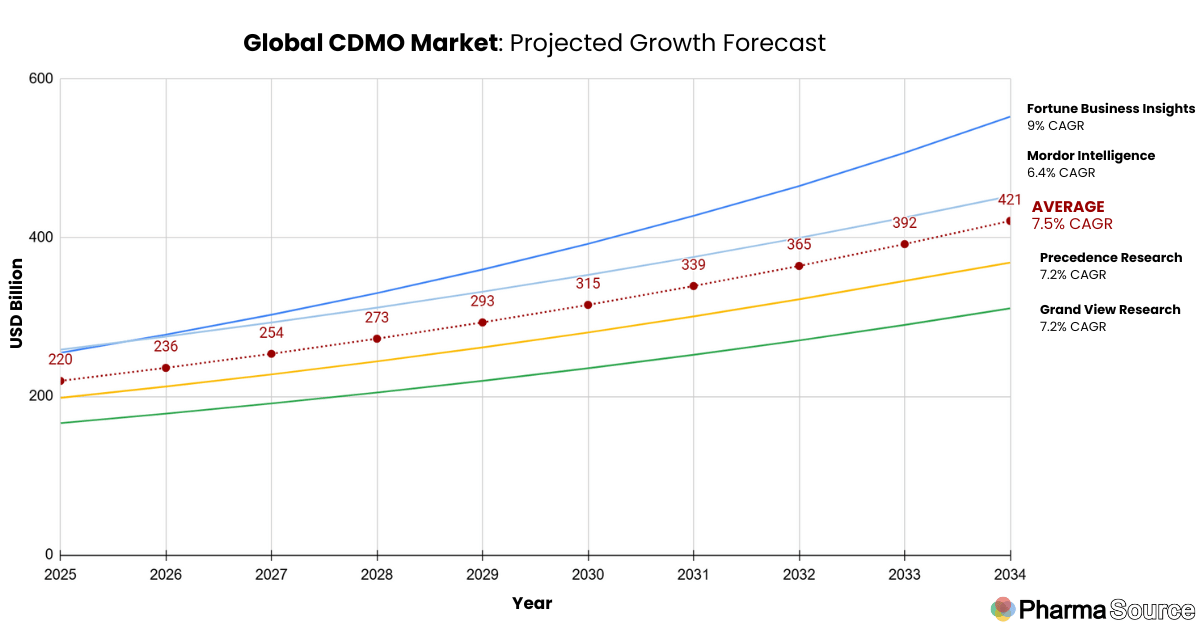
The CDMO market is valued at $220 billion USD in 2025, growing strongly at 7.5% CAGR
What Does a CDMO Do?
CDMOs offer a range of services across the pharmaceutical development and manufacturing process, including:
- Drug substance (active ingredient) manufacturing
- Drug product manufacturing (final formulation)
- Analytical testing and quality control
- Process development and optimization
- Technology transfer
- Scale-up from clinical to commercial production
- Regulatory compliance support
- Packaging and distribution
The CDMO sector continues to grow and diversify, with an ever-increasing range of companies to choose from:
Essential CDMO Resources
To help with getting the most from the CDMO sector we have created a number of useful resources:
Quality Oversight Plan for CDMOs [Download]
Request For Quote (RFQ) Checklist [DOWNLOAD]
European CDMO Landscape Infographic
Top Technologies for European CDMOs
What is a CDMO, CMO and CRO?
In the pharmaceutical industry, there are several types of service providers that companies can partner with, each offering a different range of services. Three of these are Contract Development and Manufacturing Organizations (CDMOs), Contract Manufacturing Organizations (CMOs), and Contract Research Organizations(CROs).
Key differences between them include:
Contract Research Organizations (CRO) primarily focuses on providing research services. These services can include everything from early-stage research and development to clinical trials and regulatory affairs. By partnering with a CRO, pharmaceutical companies can access specialised expertise and infrastructure for their research needs.
Contract Manufacturing Organizations (CMO), on the other hand, specialises in manufacturing services. They provide the infrastructure and expertise needed to manufacture pharmaceutical products on a large scale. This allows pharmaceutical companies to bring their products to market without having to invest in their own manufacturing facilities.
Contract Development and Manufacturing Organizations (CDMO) provides a combination of both research and manufacturing services. They offer end-to-end support for pharmaceutical companies, from the early stages of research and development all the way through to manufacturing and packaging of the final product.
By understanding these differences, pharmaceutical companies can make informed decisions about which type of organisation to partner with based on their phase and specific needs.

Read CDMOs vs CROs vs CMOs: Understanding Key Services at Each Stage of Drug Development
The Role of a CDMO in Drug Development and Manufacturing
CDMOs can support pharmaceutical companies at every step of the way, from drug development to commercial manufacturing. This allows pharmaceutical companies to focus on their core competencies, such as drug discovery and marketing, while leaving the complex tasks of drug development and manufacturing to experts.
At the early stages of drug development, a CDMO can provide services such as pre-formulation and formulation development. Pre-formulation involves studying the physical and chemical properties of a drug, which is essential for designing effective and stable formulations. Formulation development then involves creating the actual drug product, which could be in various forms such as tablets, capsules, liquids, or injectables.
Once the drug formulation is developed, the CDMO can also assist with stability studies. These studies are critical for determining the shelf life of the drug product and understanding how different environmental factors might affect its stability.
As the drug moves into the clinical trial phase, a CDMO can provide clinical trial materials. These include the investigational medicinal product (IMP) that will be administered to patients during the trial, as well as the necessary documentation to ensure regulatory compliance.
Finally, once the drug is approved for marketing, a CDMO can scale up production from clinical trial quantities to commercial-scale quantities. They can also handle packaging and distribution of the final product.
Why Pharma companies use CDMOs
Pharmaceutical companies operate in a highly competitive and regulated environment. Developing a new drug is a complex, time-consuming, and expensive process. It involves numerous stages, each with its own set of challenges, from initial research and development to clinical trials and finally, manufacturing and marketing. This is where CDMOs come in.
CDMOs offer a range of benefits to pharmaceutical companies. Here are some key reasons why pharma companies choose to partner with CDMOs:
- Speed to Market: CDMOs have the expertise and resources to expedite the drug development process, helping pharmaceutical companies bring their products to market faster.
- Cost Savings: By outsourcing the drug development and manufacturing process to a CDMO, pharmaceutical companies can achieve significant cost savings. They can avoid the high capital expenditure associated with setting up their own manufacturing facilities and reduce operational costs.
- Scalability: CDMOs offer scalable solutions, allowing pharmaceutical companies to easily scale up or down their production based on demand.
- Focus on Core Competencies: By outsourcing the complex tasks of drug development and manufacturing to a CDMO, pharmaceutical companies can focus on their core competencies such as drug discovery and marketing.
- Regulatory Compliance: CDMOs are experienced in dealing with regulatory authorities around the world and can ensure that the drug development process complies with all relevant regulations.
- Access to Advanced Technologies: Many CDMOs have access to advanced technologies and specialised equipment that may not be available in-house for many pharmaceutical companies.
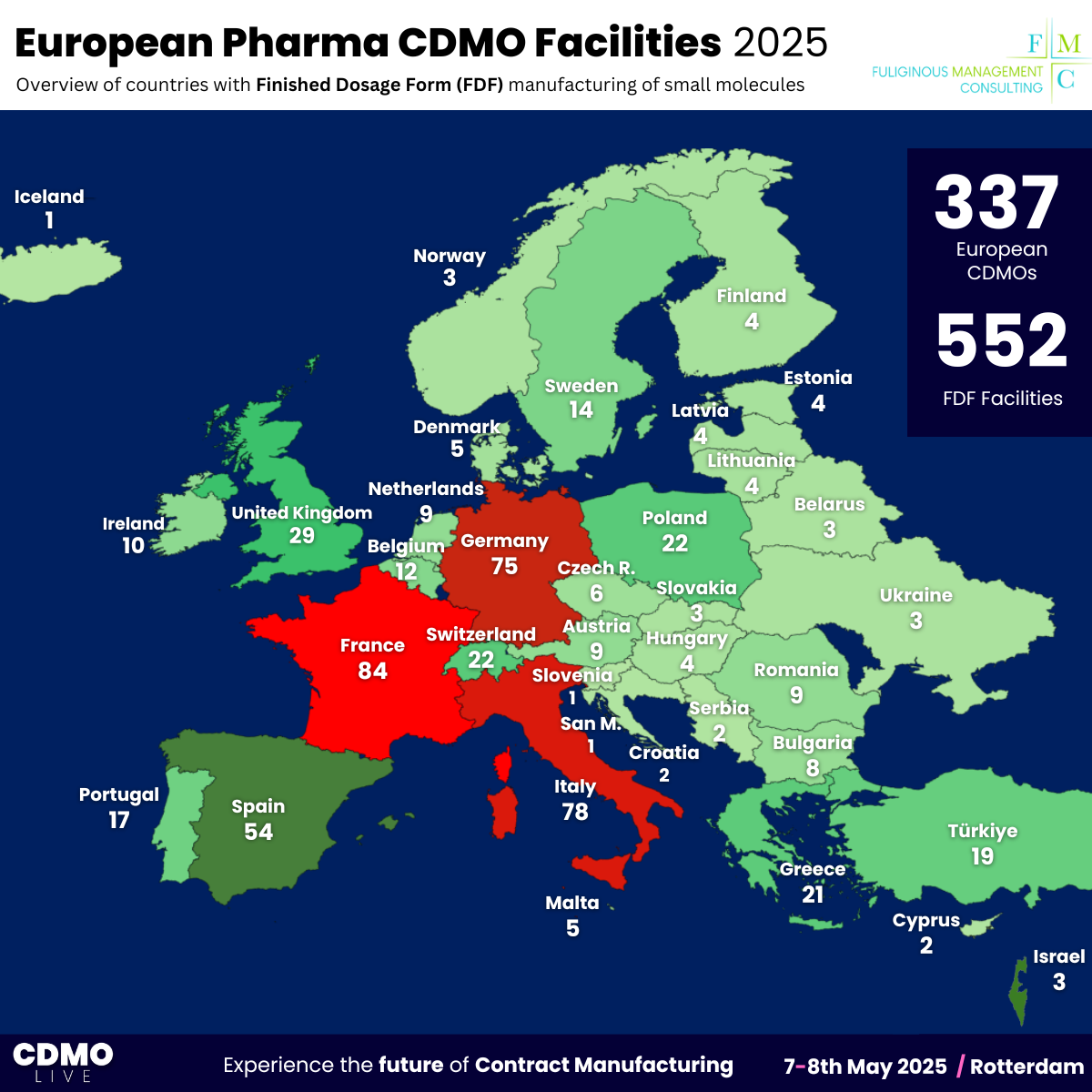
- Read our ranking of 552 FDF CDMO manufacturing sites across Europe, which reveals the countries with the most facilities in operation.
How to choose the right CDMO for your business
Selecting the right CDMO is a critical decision that can significantly impact the success of a pharmaceutical company’s drug development and manufacturing process.
A CDMO partner will likely be a long-term strategic partnership, so it is important to make sure that they are the right fit for you company in terms of size and cultural fit.
In this video, David Caron SVP CMC at Ayala Pharmaceuticals explained his framework for selecting a Contract Manufacturing partner:
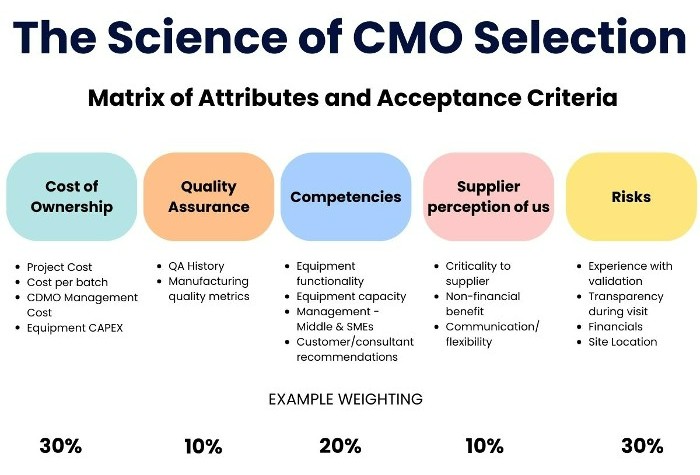
Here are some factors to consider when choosing a CDMO:
- Industry Credibility: Look for a CDMO with a strong reputation in the industry. Check their track record, client testimonials, and any industry awards or recognitions they have received.
- End-to-End Support: Ensure that the CDMO offers comprehensive services that cover all stages of drug development and manufacturing. This includes pre-formulation, formulation development, stability studies, clinical trial materials, and commercial production.
- Proactive Risk Mitigation: The CDMO should have robust risk management strategies in place to anticipate and mitigate potential challenges during the drug development and manufacturing process.
- Good Project Management: Effective project management is crucial for ensuring that projects are completed on time and within budget. Look for a CDMO with a proven track record of managing projects effectivly.
- Scalability: The CDMO should be able to scale up production quickly and efficiently to meet increasing demand.
- Trustworthiness: Trust is crucial in any partnership. The CDMO should be transparent in their operations and communication, and show a commitment to meeting their clients’ need
By considering these factors, pharmaceutical companies can choose a CDMO that not only meets their current needs but also supports their long-term goals.
Listen to Janet Hoogstraate, CEO of NorthX Biologics on the PharmaSource podcast as she explains how CDMO–biotech collaboration is shifting toward deeper, integrated partnerships that support clinical development and long-term success.