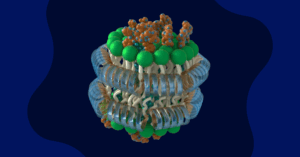
- Genprex has partnered with a contract development and manufacturing organisation (CDMO) to explore a non-viral lipid nanoparticle delivery system for its diabetes gene therapy drug candidate.
- The collaboration is separate from the company’s ongoing preclinical studies and aims to optimise construct design and improve potential re-dosing capabilities.
Genprex, Inc., a clinical-stage gene therapy company, has announced a strategic collaboration with a contract development and manufacturing organisation (CDMO) to explore a second-generation approach for delivering its diabetes gene therapy drug candidate. The research will focus on using a non-viral lipid nanoparticle delivery system as an alternative to the adeno-associated virus (AAV) method currently under investigation.
The collaboration aims to evaluate the feasibility of this delivery system and its potential advantages. “This collaboration with our CDMO partner is separate from our existing preclinical research programs and seeks to evaluate potential next-generation construct optimization,” said Ryan Confer, President and Chief Executive Officer at Genprex. He noted that a non-viral delivery system could enable re-dosing, which is not possible with viral-based approaches due to immune system responses.
Currently, Genprex is developing its GPX-002 gene therapy candidate for both Type 1 and Type 2 diabetes using an AAV vector to deliver the Pdx1 and MafA genes directly into the pancreatic duct. The therapy aims to transform alpha cells into insulin-producing beta-like cells in Type 1 diabetes, while in Type 2 diabetes, it is believed to rejuvenate and replenish exhausted beta cells. Preclinical studies have shown that GPX-002 restored normal blood glucose levels for extended periods in diabetic mouse models.
By investigating a non-viral delivery system, Genprex and its CDMO partner hope to enhance the therapy’s durability and allow for multiple administrations. Chief Medical Officer Mark Berger highlighted the significance of their preclinical findings, noting that GPX-002 had demonstrated glucose control durability of approximately 120 days in Type 1 diabetic mouse models.