Description
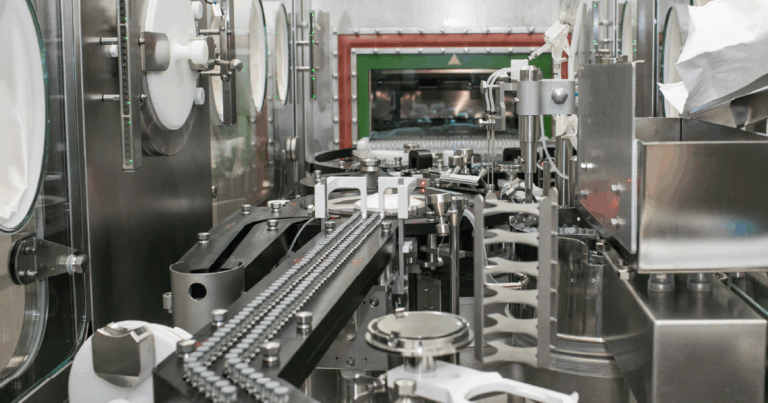
ASCIL Biopharm is a Contract Development and Manufacturing Organisation (CDMO) that is fully equipped for early-stage drug product development. The company operates a Good Manufacturing Practice (GMP) facility designed for the production of clinical batches, utilising both aseptic processes and terminal sterilisation techniques. ASCIL Biopharm specializes in filling various types of containers, including vials and syringes, and possesses extensive formulation, device, and analytical development capabilities. The company’s expertise spans a wide range of molecules, with a particular focus on peptides and biologics, such as proteins, fusion proteins, toxins, and monoclonal antibodies (mAbs). ASCIL Biopharm engages with both its own and external assets in diverse therapeutic areas including endocrine, cancer, infectious, dermatological, and ocular diseases.
Services include:
- Biologics
- Contract Development and Manufacturing (CDMO)
- Aseptic process development
- Terminal sterilisation
- Formulation development
- Analytical development
- Device development
ASCIL Biopharm continues to deliver innovative solutions to the pharmaceutical industry, ensuring high-quality support for drug product development and manufacturing. For more information, visit ascil-biopharm.com.