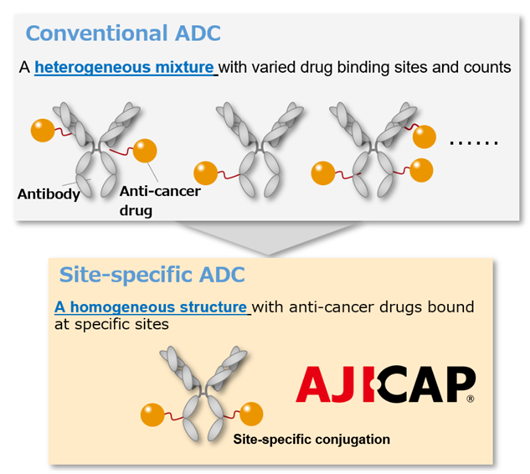
Description
Emergent Bioservices offers biopharmaceutical companies a strategic and reliable solution to their clinical and commercial supply chain needs. As a committed and reliable CDMO partner, they possess the scientific and regulatory compliance experience alongside cGMP manufacturing capabilities to support complex and challenging programmes.
Services include:
- Accelerated Stability Studies
- Analytical Services
- Assay Development
- Biologics
- Process Development
- Biosimilars
- Clinical Trials
- Chemistry (CMC)
- Manufacturing
- Contract Development and Manufacturing (CDMO)
- Contract Manufacturing (CMO)
- Downstream Processing
- Filling
- Syringe
- Vials
- Gene Therapy & Testing Protocols
- Lot Release Testing
- Lyophilization
- Aseptic Fill-Finish
- Clinical Trial Materials
- Dose Form
- Sterile
- Injectables
- Liquids
- Oligonucleotides
- Parenterals
- Small Volume
- Peptides
- Proteins
- Pre-filled
- Vaccines
- Animal Health
- Packaging
- Process Validation Studies
- Purification
- Quality QA/QC
- Stability Studies
- Stability
- ICH
- Sterile Filling
- Sterility Testing
- Technology Transfer
- Validation
- Clinical
Emergent Bioservices is dedicated to providing comprehensive support for the needs of the pharmaceutical industry. For more information, visit emergentbio.com.
Capabilities
- Accelerated Stability Studies
- Analytical Services
- Animal Health
- Asceptic Fill-Finish
- Assay Development
- Biologics
- Biosimilars
- Cell and Gene Therapy
- Chemistry (CMC)
- Clinical
- Clinical Trial Materials
- Clinical Trials
- Contract Development and Manufacturing (CDMO)
- Dose Form
- Downstream Processing
- Filling
- ICH
- Injectables
- Liquids
- Lot Release Testing
- Lyophilization
- Oligonucleotides
- Packaging
- Parenterals
- Peptides
- Process Development
- Process Validation Studies
- Proteins
- Purification
- Quality QA/QC
- Stability Studies
- Sterile
- Sterile Filling
- Sterility Testing
- Syringe
- Technology Transfer
- Vaccines
- Validation
- Vials