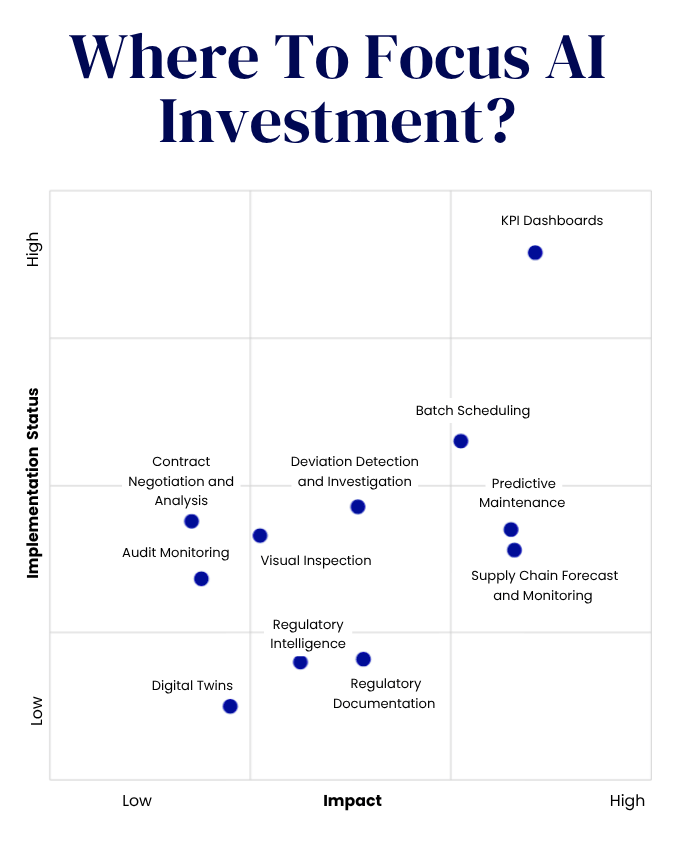
- Cytiva, a global life sciences company, has developed new ELEVECTA cell lines to address the challenges of viral vector-based gene therapies.
- The company has created three distinct cell lines for adeno-associated virus (AAV) production.
Cytiva, is making strides in the field of gene therapy. The company has developed new ELEVECTA cell lines, aiming to standardise the manufacturing process of viral vector-based gene therapies.
Emmanuel Abate, President Genomic Medicine at Cytiva said To make gene therapy a realistic option for more patients, we need to be able to standardize its manufacturing. We’ve gone to the heart of the process by creating viral vector cell lines that will make a big impact to developers of gene therapies.”
The company has leveraged its extensive expertise to create three distinct cell lines for adeno-associated virus (AAV) production. These cell lines cater to the unique objectives of various therapeutic programs, offering the flexibility to transition between cell lines as needs evolve.
The first is a transient cell line that provides speed and flexibility, enabling researchers to easily integrate it into their existing workflow. This cell line is paired with a new cell culture medium, HyClone prime expression, designed to enhance performance. The second is a packaging cell line that eases the screening of genes of interest while enabling efficiencies across clinical stages and assets. The third is a producer cell line that stably integrates all four required genes for AAV production. Production starts by simply adding an induction agent—no transfection is needed.
Abate concludes, “We now offer end-to-end solutions for viral vector manufacturing. With our new cell line offering, our customers can deliver higher quality treatments and significantly improve patient access today as well as meet the increasing demand for viral vector material tomorrow.” This development marks a significant milestone in Cytiva’s commitment to advancing gene therapy and contract manufacturing.