The pharmaceutical supply chain serves as the backbone of the healthcare industry, ensuring the timely and efficient delivery of life-saving medications to patients worldwide. At its core, the pharma supply chain encompasses a complex network of interconnected processes, stakeholders, and regulations aimed at safeguarding the quality, safety, and efficacy of pharmaceutical products.
Definition and Importance
The pharmaceutical supply chain refers to the series of interconnected activities involved in the sourcing, manufacturing, distribution, and delivery of pharmaceutical products to end-users, including patients, healthcare providers, pharmacies, and hospitals. It plays a critical role in ensuring that medications are available when needed and in the right quantities, ultimately impacting patient health outcomes.
In addition to providing access to essential medications, an efficient pharmaceutical supply chain is essential for maintaining public health, particularly during emergencies such as disease outbreaks or natural disasters. Rapid response capabilities and robust supply chain infrastructure are crucial for delivering medical supplies and treatments to affected populations in a timely manner.
Key stages and stakeholders in the supply chain
The pharmaceutical supply chain involves collaboration among various stakeholders, each playing a unique role in the process. These stakeholders include:
- Pharmaceutical suppliers and manufacturers: Companies responsible for producing pharmaceutical products, ranging from generic drugs to specialty medications. Suppliers provide of raw materials, active pharmaceutical ingredients (APIs), excipients, packaging materials, and other components necessary for drug manufacturing.
- Quality Control: Throughout the manufacturing process, rigorous quality control measures are implemented to ensure that pharmaceutical products meet predefined specifications for identity, strength, purity, and stability. Quality control testing may involve analytical testing, microbiological testing, and physical testing to assess product quality and compliance with regulatory requirements.
- Distributors and wholesalers: Entities responsible for the storage, transportation, and wholesale distribution of pharmaceutical products to pharmacies, hospitals, and healthcare facilities. Pharmaceutical products must be stored under appropriate conditions to maintain their stability, potency, and integrity throughout their shelf life. Storage and warehousing facilities must adhere to Good Distribution Practices (GDP) and Good Storage Practices (GSP), including temperature control, humidity control, and proper handling procedures to prevent product degradation and spoilage.
- Regulatory agencies: Government bodies responsible for overseeing and enforcing regulations related to pharmaceutical manufacturing, distribution, and safety.
- Healthcare providers: Hospitals, clinics, and pharmacies that dispense medications to patients and provide healthcare services.
- Patients: End-users of pharmaceutical products who rely on the supply chain for access to essential medications to manage their health conditions.
In a recent PharmaSource podcast Kearney partner, author and futurist Elouise Epstein explained that “Third-party data exchange (between partners) is the key to the future. Above all, we need to facilitate easy data exchange with suppliers.”
In her new book, How to Hack the Supply Chain, Elouise mapped out the steps that led up to having a COVID-19 injection, steps which should be made transparent and easily-accessible to patients:

Impact on the Pharmaceutical Industry
The efficiency and reliability of the pharmaceutical supply chain directly impact the overall performance and success of the pharmaceutical industry. A well-functioning supply chain can contribute to:
– Improved patient outcomes: Timely access to medications ensures that patients receive the treatments they need to manage their health conditions effectively.
– Cost savings: Optimizing supply chain processes can help reduce manufacturing, distribution, and inventory management costs, ultimately leading to lower healthcare expenses for patients and healthcare systems.
– Regulatory compliance: Adhering to regulatory requirements and quality standards is essential for maintaining product safety and compliance with global regulations.
– Competitive advantage: Companies that invest in supply chain innovation and optimization can gain a competitive edge by offering faster delivery times, higher product quality, and enhanced customer service.
Pharma Supply Chain Dynamics
The pharmaceutical supply chain operates within a dynamic environment characterised by evolving market trends, regulatory requirements, technological advancements, and geopolitical factors. Understanding these dynamics is essential for stakeholders to navigate challenges effectively and capitalise on emerging opportunities.
Market Trends: Shifts in consumer preferences, healthcare policies, and disease prevalence can influence demand patterns and product mix within the pharmaceutical industry. For example, the growing prevalence of chronic diseases, such as diabetes and cardiovascular conditions, has fueled demand for specialised medications and personalised treatment options.
Regulatory Requirements: Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), play a crucial role in overseeing pharmaceutical manufacturing, distribution, and safety standards. Compliance with regulatory requirements is paramount for ensuring product quality, safety, and efficacy throughout the supply chain.
Technological Advancements: The adoption of advanced technologies, such as artificial intelligence (AI), blockchain, and Internet of Things (IoT) devices, is revolutionizing supply chain management practices in the pharmaceutical industry. These technologies enable real-time monitoring, data analytics, and process automation, leading to enhanced efficiency, transparency, and traceability.
Geopolitical Factors: Political instability, trade tensions, and natural disasters can disrupt global supply chains, causing supply shortages, delays, and price fluctuations. Geopolitical risks, such as trade tariffs and export restrictions, underscore the importance of diversifying sourcing strategies and building resilient supply chain networks.
Globalisation and Supply Chain Complexity
The pharmaceutical supply chain is inherently global, with raw materials, manufacturing facilities, and distribution channels spanning multiple countries and regions. Globalisation has led to increased supply chain complexity, as companies navigate diverse regulatory environments, cultural differences, and logistical challenges.
Supply Chain Localisation: Despite the benefits of globalisation, there is a growing trend towards supply chain localisation to mitigate risks associated with long-distance transportation, geopolitical instability, and regulatory uncertainties. Localisation strategies involve establishing regional manufacturing hubs, sourcing suppliers locally, and partnering with regional distributors to enhance agility and responsiveness.
Supply Chain Visibility: Achieving end-to-end visibility and transparency across the supply chain is a top priority for pharmaceutical companies seeking to optimise inventory management, reduce lead times, and mitigate risks. Advanced tracking and tracing technologies, such as RFID tags, barcodes, and serial number tracking, enable real-time monitoring of product movement and status throughout the supply chain.
Collaborative Partnerships: Collaboration among supply chain partners, including manufacturers, suppliers, distributors, and logistics providers, is essential for driving innovation, sharing best practices, and addressing common challenges. Strategic partnerships can facilitate knowledge exchange, resource pooling, and joint problem-solving initiatives to improve supply chain efficiency and resilience.
Challenges in Supply Chain and Logistics
The pharmaceutical supply chain operates within a challenging environment characterised by regulatory requirements, product integrity concerns, globalisation, and demand variability. In this chapter, we will explore the key challenges faced by stakeholders in supply and logistics management and discuss strategies for addressing them effectively.
Regulatory Hurdles and Compliance Requirements
Regulatory compliance is a cornerstone of pharmaceutical supply chain management, with stringent requirements imposed by regulatory agencies to ensure product safety, quality, and efficacy. Compliance challenges can arise from:
– Evolving Regulations: Regulatory requirements are subject to frequent updates and revisions, necessitating ongoing monitoring and adaptation to ensure compliance with changing standards.
– Cross-Border Trade: International trade regulations, including import/export restrictions and customs clearance procedures, can pose challenges for companies operating in multiple jurisdictions.
– Serialisation and Traceability: Regulatory mandates for product serialisation and traceability require companies to implement robust track-and-trace systems to monitor product movement and authenticity throughout the supply chain.
Addressing regulatory hurdles requires proactive engagement with regulatory agencies, investment in compliance management systems, and collaboration with supply chain partners to ensure adherence to applicable regulations.
Counterfeit Drugs and Product Integrity Concerns
“Let’s face it, in the pharma supply chain there are bad guys and we’re always trying to stay one step ahead. There’s a big financial gain to be made from counterfeiting drugs”
Jim Fries, CEO Rx-360
Counterfeit drugs pose a significant threat to public health and safety, undermining trust in the pharmaceutical supply chain and exposing patients to potentially harmful products. Common challenges related to counterfeit drugs and product integrity include:
– Supply Chain Vulnerabilities: Complex global supply chains increase the risk of counterfeit drugs entering the supply chain through unauthorised channels or counterfeiters exploiting vulnerabilities in the distribution network.
– Authentication Technologies: Implementing robust authentication technologies, such as tamper-evident packaging, holographic labels, and serial number verification, can help deter counterfeiters and protect product integrity.
– Regulatory Compliance: Compliance with serialisation and track-and-trace regulations is essential for preventing counterfeit drugs and ensuring product authenticity throughout the supply chain.
Combating counterfeit drugs requires a multi-faceted approach involving collaboration among stakeholders, adoption of advanced authentication technologies, and regulatory enforcement efforts to deter illicit activities.
In this workshop for PharmaSource, Jim spoke about the importance of protecting supply chain integrity.
“It really comes down to making sure that the audit foundation is solid. Make sure that the supplier that you’re working with checks the necessary quality boxes. Make sure the audit is recent and that it comes from the root source and wasn’t passed along from, from one auditor to another. “
“If you’re a procurement person at a pharma company, make sure that you have open lines of communication with your quality departments. Those two departments have to work hand in hand to help each other.”
Watch the full presentation below
Global supply chains and distribution complexities
Globalisation has transformed the pharmaceutical supply chain, enabling companies to access new markets and leverage cost-effective manufacturing and sourcing opportunities. However, globalisation also introduces complexities and challenges, including:
– Supply Chain Disruptions: Geopolitical tensions, natural disasters, and global health crises can disrupt supply chain operations, causing delays, shortages, and supply chain inefficiencies.
– Cultural and Regulatory Differences: Variations in cultural norms, language barriers, and regulatory requirements across countries and regions can complicate supply chain management and compliance efforts.
– Transportation and Logistics Challenges: Long-distance transportation, customs clearance procedures, and infrastructure limitations in developing countries can impact transportation costs, lead times, and supply chain agility.
Mitigating the challenges of globalisation requires a comprehensive risk management strategy, diversification of sourcing and manufacturing locations, and investment in logistics infrastructure and contingency planning.
Inventory Management and Demand Forecasting Challenges
Effective inventory management and demand forecasting are essential for optimising supply chain performance and minimising inventory carrying costs. Common challenges in inventory management and demand forecasting include:
– Demand Variability: Fluctuations in demand patterns, seasonality, and market trends can complicate demand forecasting efforts and lead to inventory imbalances.
– Stockouts and Excess Inventory: Stockouts can result in lost sales and patient dissatisfaction, while excess inventory ties up working capital and increases carrying costs.
– Data Accuracy and Visibility: Limited visibility into inventory levels, inaccurate demand forecasts, and data silos can hinder decision-making and lead to suboptimal inventory management practices.
Addressing inventory management and demand forecasting challenges requires leveraging advanced analytics, demand sensing technologies, and collaboration with supply chain partners to improve forecast accuracy, optimise inventory levels, and enhance supply chain agility.
Key Processes in the Pharmaceutical Supply Chain
Within each stage of the pharmaceutical supply chain, several key processes and activities take place to ensure the efficient flow of materials and information. These processes include:
– Demand Planning and Forecasting: Forecasting demand for pharmaceutical products is essential for optimising inventory levels, production schedules, and distribution plans. Demand planning involves analysing historical sales data, market trends, and customer preferences to anticipate future demand and align supply chain operations accordingly.
– Order Fulfilment: Order fulfilment processes involve receiving customer orders, picking, packing, and shipping products, and updating inventory records to reflect transactional data accurately. Efficient order fulfilment requires coordination between sales, logistics, and inventory management teams to meet customer expectations for product availability and delivery times.
– Regulatory Compliance: Compliance with regulatory requirements is paramount throughout the pharmaceutical supply chain to ensure product safety, quality, and efficacy. Regulatory compliance activities may include product registration, labeling, documentation, and reporting to regulatory agencies to demonstrate compliance with applicable regulations.
– Traceability and Serialisation: Traceability and serialisation technologies enable tracking and tracing of pharmaceutical products throughout the supply chain, from manufacturing to distribution to end-users. Serialisation involves assigning unique identifiers, such as serial numbers or barcodes, to individual product units to enable traceability and authentication.
Stakeholders in the Pharmaceutical Supply Chain
The pharmaceutical supply chain involves collaboration among various stakeholders, each contributing to the movement and management of pharmaceutical products. Key stakeholders in the pharmaceutical supply chain include:
– Pharmaceutical Manufacturers: Companies responsible for producing pharmaceutical products, including branded drugs, generic drugs, and biologics.
– Suppliers: Providers of raw materials, active pharmaceutical ingredients (APIs), excipients, packaging materials, and other components used in drug manufacturing.
– Distributors and Wholesalers: Entities responsible for the storage, transportation, and wholesale distribution of pharmaceutical products to pharmacies, hospitals, clinics, and healthcare providers.
– Healthcare Providers: Hospitals, clinics, pharmacies, and healthcare facilities that dispense medications to patients and provide healthcare services.
– Regulatory Agencies: Government bodies responsible for overseeing and enforcing regulations related to pharmaceutical manufacturing, distribution, and safety.
– Patients: End-users of pharmaceutical products who rely on the supply chain for access to essential medications to manage their health conditions effectively.
Shifting Trends in Pharma Supply Chain for New Forms of Treatments
The pharmaceutical supply chain is constantly evolving to meet the demands of an ever-changing healthcare landscape. In recent years, there has been a notable shift towards the development and delivery of new forms of treatments, including specialty drugs, biologics, and personalised medications.
Specialty Drugs and Biologics
Specialty drugs and biologics represent a growing segment of the pharmaceutical market, offering targeted therapies for complex and chronic diseases such as cancer, autoimmune disorders, and rare genetic conditions. Unlike traditional small-molecule drugs, specialty drugs and biologics are often complex molecules derived from living organisms, requiring specialised manufacturing processes and storage conditions.
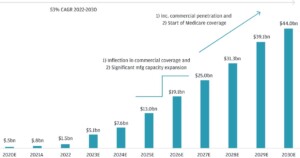
Biopharmaceuticals already make up over 30% of the total pharma market. This share is forecast to reach 41% by 2028, requiring strict temperature control from production through final delivery to maintain stability.
In a recent interview with DHL’s Fernanda Teles, Head of Global Clinical Logistics for DHL Supply Chain, she explained how the growing Cell and gene therapy market size (pictured below)will require expanded and upgraded cold chain capabilities.

– Manufacturing Challenges: The production of specialty drugs and biologics presents unique challenges due to their complex molecular structures and sensitivity to environmental conditions. Manufacturers must adhere to stringent quality control measures and invest in specialised facilities and equipment to ensure product purity, potency, and stability.
– Distribution Considerations: Specialty drugs and biologics require specialised handling and storage throughout the distribution process to maintain their efficacy and safety. Cold chain logistics, including temperature-controlled transportation and storage, are essential for preserving the integrity of these medications and preventing degradation.
– Patient Access: Ensuring patient access to specialty drugs and biologics can be challenging due to their high cost, limited distribution channels, and reimbursement barriers. Collaboration between manufacturers, healthcare providers, payers, and patient advocacy groups is essential for overcoming these barriers and improving patient access to innovative therapies.
Personalised Medication
Advancements in genomic medicine and precision therapy have led to the development of personalised medications tailored to individual patient characteristics, such as genetic makeup, biomarker expression, and disease subtype. Personalised medications offer the potential for improved treatment outcomes, reduced adverse effects, and enhanced patient adherence.
– Pharmacogenomics: Pharmacogenomic testing enables healthcare providers to identify genetic variations that may impact an individual’s response to specific medications. By incorporating genetic information into treatment decisions, healthcare providers can optimise medication selection, dosing, and monitoring to maximise therapeutic benefits and minimise risks.
– Compounding Pharmacy: Compounding pharmacies play a crucial role in the provision of personalised medications, offering customised formulations and dosage forms to meet the unique needs of individual patients. Compounded medications may be prescribed for patients with allergies, sensitivities, or other special requirements that cannot be met by commercially available products.
– Regulatory Considerations: Regulatory oversight of personalised medications varies by jurisdiction, with regulations governing compounding practices, patient safety, and product quality. Healthcare providers and compounding pharmacies must adhere to applicable regulations and quality standards to ensure the safety and efficacy of personalised medications.
Track and Trace Technologies
The rise of counterfeit drugs and supply chain security concerns has prompted the adoption of track and trace technologies to enhance product authentication, traceability, and security throughout the pharmaceutical supply chain. Track and trace technologies enable stakeholders to monitor the movement of pharmaceutical products from manufacturing facilities to end-users, ensuring transparency and accountability at every stage of the supply chain.
– Serialisation: Serialisation involves assigning unique identifiers, such as serial numbers or barcodes, to individual product units to enable traceability and authentication. Serialised products can be tracked and traced throughout the supply chain, allowing stakeholders to verify product authenticity and detect counterfeit drugs.
– Track and Trace Systems: Track and trace systems leverage serialisation data to track the movement of pharmaceutical products in real-time and monitor key supply chain metrics such as inventory levels, delivery times, and product recalls. Advanced track and trace systems may incorporate technologies such as RFID tags, QR codes, and blockchain to enhance visibility and transparency.
– Regulatory Mandates: Regulatory agencies worldwide have implemented serialisation and track and trace requirements to combat counterfeit drugs and improve supply chain security. Compliance with regulatory mandates is essential for pharmaceutical companies to maintain market access and ensure product safety and integrity.
Cold Chain Logistics
The rise of biologics, vaccines, and other temperature-sensitive pharmaceuticals has emphasised the importance of cold chain logistics in the pharmaceutical supply chain. Cold chain logistics involves the transportation, storage, and handling of temperature-sensitive products within a controlled temperature range to maintain their efficacy and safety.
– Temperature Requirements: Cold chain logistics typically involves maintaining product temperatures within a specified range, often between 2°C to 8°C (refrigerated) or -20°C to -80°C (frozen), depending on the requirements of the specific product. Deviations from these temperature ranges can compromise product stability and efficacy.
– Temperature Monitoring: Continuous temperature monitoring is essential throughout the cold chain to ensure product integrity and compliance with regulatory requirements. Temperature monitoring devices, such as data loggers, temperature sensors, and temperature-sensitive labels, provide real-time data on temperature excursions and deviations.
– Packaging Solutions: Cold chain packaging solutions, including insulated containers, thermal blankets, and refrigerated containers, help maintain product temperatures during transportation and storage. Advanced packaging technologies, such as phase-change materials and vacuum-insulated panels, offer enhanced thermal protection and longer duration for temperature-controlled shipments.
Patient-Centric Supply Chain
As healthcare systems transition towards patient-centric care models, there is a growing emphasis on personalised medicine, patient engagement, and value-based healthcare delivery. The patient-centric supply chain aims to optimise supply chain processes and resources to meet the individual needs and preferences of patients while ensuring timely access to medications and healthcare services.
– Patient Engagement: Engaging patients in the supply chain process can improve medication adherence, treatment outcomes, and patient satisfaction. Patient-centric supply chain initiatives may involve patient education, medication counselling, remote monitoring, and support services to empower patients and promote self-management of their health conditions.
– Last-Mile Delivery: Last-mile delivery refers to the final leg of the supply chain, where pharmaceutical products are delivered directly to patients’ homes or healthcare facilities. Last-mile delivery solutions, such as courier services, home healthcare providers, and telemedicine platforms, enable convenient access to medications and healthcare services, particularly for patients with mobility limitations or chronic conditions.
– Supply Chain Visibility: Enhancing supply chain visibility and transparency is essential for patient-centric supply chain management. Real-time tracking and monitoring of product shipments, inventory levels, and delivery status enable stakeholders to anticipate patient needs, proactively address supply chain disruptions, and provide timely interventions to ensure continuity of care.
Sustainability in the Pharmaceutical Supply Chain
As concerns about environmental sustainability continue to grow, there is increasing pressure on pharmaceutical companies to adopt sustainable practices throughout their supply chains. Sustainable supply chain management involves minimising environmental impact, reducing carbon emissions, and promoting social responsibility while maintaining product quality and supply chain efficiency.
Sustainable Packaging Solutions
Packaging plays a significant role in the pharmaceutical supply chain, ensuring product integrity, safety, and compliance. Implementing sustainable packaging solutions is crucial for reducing environmental impact and minimising waste throughout the product lifecycle.
– Eco-Friendly Materials: Pharmaceutical companies can explore the use of eco-friendly materials, such as recyclable plastics, biodegradable polymers, and compostable packaging, to reduce the environmental footprint of packaging materials. Sustainable packaging alternatives help minimise waste generation and promote circular economy principles.
– Lightweight Design: Lightweight packaging design reduces material usage, transportation costs, and carbon emissions associated with packaging production and distribution. Optimising packaging dimensions, reducing excess packaging, and maximising space utilisation in shipping containers contribute to resource efficiency and waste reduction.
– Recyclability and Reusability: Designing packaging materials for recyclability and reusability facilitates material recovery and promotes closed-loop recycling processes. Pharmaceutical companies can collaborate with packaging suppliers to develop packaging solutions that are compatible with existing recycling infrastructure and promote consumer participation in recycling programs.
Green Logistics and Transportation
Logistics and transportation activities account for a significant portion of greenhouse gas emissions in the pharmaceutical supply chain. Implementing green logistics and transportation practices is essential for reducing carbon footprint, improving fuel efficiency, and mitigating environmental impact.
– Mode Shifting: Shifting freight transportation from road to rail or sea transport modes reduces carbon emissions, congestion, and fuel consumption. Intermodal transportation solutions, such as rail-road or sea-road combinations, offer cost-effective and environmentally sustainable alternatives for long-haul shipments.
– Route Optimization: Optimising transportation routes, vehicle routing, and delivery schedules minimises fuel consumption, reduces vehicle mileage, and lowers emissions. Advanced route optimization software utilises real-time traffic data, weather forecasts, and vehicle telemetry to identify the most fuel-efficient and environmentally friendly routes.
– Alternative Fuels and Electric Vehicles: Transitioning to alternative fuels, such as biodiesel, compressed natural gas (CNG), or electric vehicles (EVs), reduces dependence on fossil fuels and lowers carbon emissions from transportation operations. Electric vehicles offer zero-emission transportation solutions for last-mile delivery and urban logistics, contributing to cleaner air and healthier communities.
Renewable Energy Adoption
Pharmaceutical manufacturing facilities consume significant amounts of energy, contributing to greenhouse gas emissions and environmental impact. Adopting renewable energy sources is essential for reducing carbon footprint, improving energy efficiency, and achieving sustainability goals.
– Solar Power: Installing solar photovoltaic (PV) systems on rooftops and vacant land adjacent to manufacturing facilities generates clean, renewable energy and reduces reliance on grid electricity. Solar energy systems can offset a portion of electricity consumption, lower energy costs, and contribute to renewable energy targets.
– Wind Power: Onshore and offshore wind farms offer sustainable sources of electricity for pharmaceutical manufacturing operations. Wind power generation provides reliable, renewable energy supply, reduces dependence on fossil fuels, and mitigates environmental impact associated with conventional energy sources.
– Energy Efficiency Measures: Implementing energy efficiency measures, such as LED lighting, HVAC system upgrades, and energy management systems, improves energy efficiency, reduces energy consumption, and lowers operating costs in manufacturing facilities. Energy efficiency investments yield long-term savings and contribute to sustainability objectives by reducing greenhouse gas emissions.
Ethical Sourcing and Social Responsibility (ESG)
Ethical sourcing practices encompass responsible procurement of raw materials, including active pharmaceutical ingredients (APIs), excipients, and packaging materials, to ensure ethical labor practices, environmental sustainability, and social responsibility.
– Supplier Code of Conduct: Pharmaceutical companies establish supplier codes of conduct that outline expectations for ethical behaviour, labour standards, environmental stewardship, and human rights. Supplier codes of conduct set forth principles and guidelines for responsible sourcing, fair labour practices, and sustainable supply chain management.
– Supplier Audits and Assessments: Pharmaceutical companies conduct supplier audits and assessments to evaluate supplier compliance with ethical sourcing standards, regulatory requirements, and industry best practices. Supplier audits assess factors such as labour conditions, environmental impact, supply chain transparency, and adherence to ethical business practices.
– Ethical Certifications and Labels: Pharmaceutical companies may require suppliers to obtain ethical certifications and labels, such as Fair Trade certification, Responsible Source certification, or Ethical Trade Initiative (ETI) membership, to demonstrate compliance with ethical sourcing standards and commitment to social responsibility.
Corporate Social Responsibility (CSR) Initiatives
Corporate social responsibility (CSR) initiatives demonstrate pharmaceutical companies’ commitment to environmental sustainability, community engagement, and philanthropy. CSR programs aim to create positive social and environmental impact, address societal challenges, and promote ethical business practices.
– Environmental Sustainability: Pharmaceutical companies implement environmental sustainability initiatives to reduce carbon footprint, minimise waste generation, and conserve natural resources. CSR programs may include initiatives such as renewable energy adoption, waste reduction, water conservation, and biodiversity conservation to promote environmental stewardship.
– Community Engagement and Philanthropy: Pharmaceutical companies engage with local communities and support social causes through philanthropic initiatives, charitable donations, and community outreach programs. CSR programs may focus on initiatives such as healthcare access, education, poverty alleviation, disaster relief, and humanitarian aid to improve quality of life and support sustainable development in communities.
– Stakeholder Engagement and Transparency: Pharmaceutical companies engage with stakeholders, including employees, customers, investors, regulators, and civil society organisations, to promote transparency, accountability, and ethical business practices. CSR initiatives foster dialogue, collaboration, and trust among stakeholders, enabling stakeholders to contribute to CSR priorities, provide feedback, and hold companies accountable for their social and environmental impact.
Collaboration among supply chain partners is critical for driving sustainability initiatives and promoting responsible sourcing practices. Companies can work with suppliers to identify opportunities for waste reduction, energy conservation, and resource optimization throughout the supply chain. Collaborative efforts, such as supplier engagement programs and sustainability audits, foster transparency, accountability, and continuous improvement in sustainability performance.
The Role of Technology in Optimising Supply Chain Operations
Technology plays a crucial role in optimising supply chain operations within the pharmaceutical industry by driving efficiency, transparency, and innovation.
Speaking to PharmaSource, Elouise Epstein explained that 21st supply chain technology platform should based around cloud technologies that are able to facilitate data exchange with third-parties, rather than the Enterprise Resource Planning (ERP) systems that currently represent the foundation of most pharma supply chains.
The diagram below represents Elouise’s vision for the various applications that will need to be plugged into the new data foundation, covering all stages of sourcing, making, delivery and planning.

Blockchain Technology
Blockchain technology has gained traction in the pharmaceutical industry as a promising solution for enhancing supply chain visibility, traceability, and security. Blockchain is a decentralised, immutable ledger that records transactions across a network of computers, providing a transparent and tamper-proof record of asset movement and ownership.
– Supply Chain Transparency: Blockchain enables real-time visibility into the movement of pharmaceutical products from manufacturing facilities to end-users, allowing stakeholders to track and trace product batches, verify authenticity, and ensure compliance with regulatory requirements.
– Product Authentication: Blockchain can be used to create unique digital fingerprints, or “digital twins,” for pharmaceutical products, enabling stakeholders to verify product authenticity and prevent counterfeit drugs from entering the supply chain.
– Data Integrity and Security: Blockchain’s decentralised architecture and cryptographic algorithms ensure data integrity and security, protecting sensitive information from unauthorised access, tampering, or manipulation.
– Smart Contracts: Smart contracts are self-executing contracts with predefined terms and conditions encoded into the blockchain. In the pharmaceutical supply chain, smart contracts can automate transactional processes, such as payment settlements, regulatory compliance checks, and product recalls, based on predefined rules and triggers.
Internet of Things (IoT) Devices
The Internet of Things (IoT) refers to the network of interconnected devices embedded with sensors, actuators, and communication technologies that enable them to collect, exchange, and analyse data in real-time. In the pharmaceutical supply chain, IoT devices play a vital role in monitoring product conditions, tracking shipments, and optimising inventory management.
– Temperature Monitoring: IoT-enabled temperature sensors can monitor product temperatures during transportation and storage, providing real-time alerts and notifications in the event of temperature excursions or deviations from predefined thresholds.
– Asset Tracking: IoT devices, such as RFID tags, GPS trackers, and barcode scanners, enable stakeholders to track the movement and location of pharmaceutical products, assets, and inventory throughout the supply chain.
– Predictive Maintenance: IoT-enabled predictive maintenance solutions can monitor the condition of equipment, machinery, and infrastructure in manufacturing facilities and distribution centres, predicting potential failures and scheduling maintenance activities proactively to prevent downtime and ensure operational efficiency.
– Remote Monitoring: IoT devices can remotely monitor environmental conditions, equipment performance, and supply chain processes, providing stakeholders with actionable insights and enabling proactive decision-making to optimise supply chain operations and address potential issues before they escalate.
Artificial Intelligence (AI) and Machine Learning
Artificial intelligence (AI) and machine learning algorithms are increasingly being leveraged in the pharmaceutical supply chain to analyse large datasets, identify patterns, and make data-driven predictions and recommendations. AI-powered solutions can enhance demand forecasting, inventory optimisation, and decision-making processes across the supply chain.
– Demand Forecasting: AI algorithms analyse historical sales data, market trends, and external factors to generate accurate demand forecasts, enabling stakeholders to optimise inventory levels, production schedules, and distribution plans to meet customer demand while minimising stockouts and excess inventory.
– Inventory Optimisation: Machine learning algorithms can analyse inventory data, demand patterns, and lead times to optimise inventory levels, safety stock, and reorder points, reducing carrying costs, improving inventory turnover, and ensuring product availability.
– Supply Chain Risk Management: AI-powered risk management solutions can analyse supply chain data, identify potential risks and disruptions, and recommend mitigation strategies to minimise the impact of unforeseen events such as natural disasters, geopolitical tensions, or supplier disruptions.
– Route Optimisation: AI algorithms can optimise transportation routes, vehicle scheduling, and delivery routes to minimise fuel consumption, reduce transportation costs, and improve delivery efficiency, while ensuring timely and reliable delivery of pharmaceutical products to end-users.
Ensuring Compliance and Security in the Pharma Supply Chain
Compliance and security are paramount in the pharmaceutical supply chain to ensure the safety, quality, and integrity of pharmaceutical products.Regulatory frameworks govern pharmaceutical supply chain operations, with best practices for maintaining product integrity, and strategies for preventing counterfeit drugs and ensuring regulatory compliance.
Regulatory Frameworks
Regulatory agencies worldwide impose strict regulations and guidelines to govern pharmaceutical manufacturing, distribution, and safety standards. Compliance with these regulations is essential for pharmaceutical companies to maintain market access, protect public health, and ensure patient safety.
– Good Manufacturing Practices (GMP): GMP regulations outline the minimum requirements for the manufacturing, processing, packaging, and storage of pharmaceutical products to ensure their safety, quality, and efficacy. GMP compliance involves implementing quality management systems, standardised operating procedures, and quality control measures to maintain product consistency and meet regulatory requirements.
– Good Distribution Practices (GDP): GDP regulations govern the distribution and transportation of pharmaceutical products, ensuring that they are handled, stored, and transported under appropriate conditions to maintain their quality, safety, and efficacy throughout the supply chain. GDP compliance involves establishing temperature-controlled storage facilities, maintaining accurate inventory records, and implementing traceability systems to track product movement and storage conditions.
– Serialization and Track & Trace Requirements: Regulatory agencies require pharmaceutical companies to implement serialisation and track & trace systems to monitor the movement of pharmaceutical products and prevent counterfeit drugs from entering the supply chain. Serialisation involves assigning unique identifiers, such as serial numbers or barcodes, to individual product units, enabling stakeholders to track their movement and verify authenticity throughout the supply chain.
Product Integrity and Counterfeit Prevention
Maintaining product integrity and preventing counterfeit drugs are critical priorities in the pharmaceutical supply chain to protect patient safety and uphold public trust in the industry. Pharmaceutical companies must implement robust security measures and authentication technologies to safeguard product integrity and prevent counterfeit drugs from infiltrating the supply chain.
– Authentication Technologies: Pharmaceutical companies utilise various authentication technologies, such as tamper-evident packaging, holographic labels, and covert markings, to verify product authenticity and prevent counterfeiting. These technologies enable stakeholders to authenticate pharmaceutical products and detect counterfeit or adulterated drugs.
– Supply Chain Security: Securing the pharmaceutical supply chain against counterfeit drugs requires collaboration among stakeholders, including manufacturers, distributors, regulators, and law enforcement agencies. Supply chain security measures may include implementing track & trace systems, conducting risk assessments, conducting supplier audits, and enforcing regulatory compliance to prevent unauthorised access, diversion, or tampering.
– Regulatory Compliance Audits: Regulatory agencies conduct periodic inspections and audits of pharmaceutical manufacturing facilities, distribution centers, and supply chain partners to assess compliance with regulatory requirements and ensure adherence to quality standards. Companies must maintain comprehensive documentation, records, and quality management systems to demonstrate compliance with regulatory requirements and facilitate regulatory audits.
Collaboration and Transparency
Collaboration and transparency among supply chain partners are essential for ensuring compliance and security in the pharmaceutical supply chain. By fostering open communication, sharing information, and collaborating on risk management and mitigation strategies, stakeholders can enhance supply chain resilience, address compliance challenges, and strengthen security measures.
– Supply Chain Collaboration: Pharmaceutical companies collaborate with suppliers, distributors, logistics providers, and regulatory agencies to implement supply chain security measures, ensure regulatory compliance, and address compliance challenges effectively. Collaborative initiatives may include information sharing, joint training programs, and risk assessment workshops to identify and mitigate supply chain risks.
– Transparency and Traceability: Transparency and traceability enable stakeholders to monitor product movement, verify authenticity, and track the flow of pharmaceutical products throughout the supply chain. By implementing serialisation and track & trace systems, stakeholders can enhance supply chain visibility, detect counterfeit drugs, and ensure regulatory compliance.
Future Trends and Innovations in the Pharma Supply Chain
The pharmaceutical supply chain is continually evolving in response to advancements in technology, shifting market dynamics, and emerging healthcare trends. In this chapter, we will explore future trends and innovations that are shaping the future of the pharma supply chain and driving transformative change in the industry.
Digitalization and Industry 4.0
The digitalization of the pharmaceutical supply chain, often referred to as Industry 4.0, is revolutionising supply chain management practices and enabling stakeholders to leverage data-driven insights, automation, and connectivity to enhance efficiency, agility, and innovation.
– Advanced Analytics: Advanced analytics and big data analytics enable stakeholders to analyse vast amounts of supply chain data, identify patterns, and extract actionable insights to optimise inventory management, demand forecasting, and decision-making processes.
– Artificial Intelligence (AI) and Machine Learning: AI and machine learning algorithms are increasingly being used to automate repetitive tasks, improve forecasting accuracy, and optimise supply chain operations. AI-powered solutions can analyse data in real-time, identify anomalies, and recommend predictive actions to address supply chain disruptions proactively.
– Robotics and Automation: Robotics and automation technologies, such as autonomous vehicles, robotic process automation (RPA), and robotic warehouse systems, are transforming manufacturing, warehousing, and distribution operations, reducing labour costs, improving efficiency, and enhancing safety.
– Digital Twins: Digital twins are virtual representations of physical assets, processes, and systems that enable stakeholders to simulate, monitor, and optimise supply chain operations in real-time. Digital twins provide a digital replica of the physical supply chain, enabling stakeholders to visualise performance, identify inefficiencies, and test scenarios to improve decision-making and agility.
Personalized Medicine and Precision Supply Chain
The rise of personalised medicine and precision therapy is driving the need for a more agile and responsive supply chain that can accommodate individual patient needs, preferences, and treatment regimens.
– Patient-Centric Supply Chain: The patient-centric supply chain focuses on delivering personalised medications and healthcare services tailored to individual patient characteristics, preferences, and treatment requirements. By leveraging data analytics, digital technologies, and patient engagement strategies, stakeholders can optimise supply chain processes to meet the unique needs of patients and improve treatment outcomes.
– Just-in-Time Manufacturing: Just-in-time (JIT) manufacturing and on-demand production enable pharmaceutical companies to produce medications in small batches, based on real-time demand signals and patient prescriptions. JIT manufacturing reduces inventory holding costs, minimises wastage, and enables faster response to changing market conditions and patient needs.
– Agile Supply Chain Networks: Agile supply chain networks are designed to respond quickly to changes in demand, supply, and market dynamics, enabling stakeholders to adapt production schedules, distribution plans, and inventory levels in real-time. Agile supply chains prioritise flexibility, responsiveness, and collaboration among supply chain partners to meet customer demand while minimising lead times and inventory costs.
Sustainability and Green Supply Chain
The pharmaceutical industry is increasingly embracing sustainability practices and green supply chain initiatives to reduce environmental impact, promote social responsibility, and meet stakeholder expectations for corporate sustainability.
– Renewable Energy Sources: Pharmaceutical companies are investing in renewable energy sources, such as solar, wind, and hydroelectric power, to reduce greenhouse gas emissions, lower energy costs, and minimise reliance on fossil fuels.
– Eco-Friendly Packaging: Eco-friendly packaging solutions, such as recyclable materials, biodegradable plastics, and compostable packaging, are being adopted to reduce packaging waste and minimise environmental footprint throughout the supply chain.
– Sustainable Sourcing Practices: Pharmaceutical companies are implementing sustainable sourcing practices to ensure responsible procurement of raw materials, including active pharmaceutical ingredients (APIs), excipients, and packaging materials. Sustainable sourcing initiatives promote ethical labour practices, environmental stewardship, and biodiversity conservation in supply chain operations.
Challenges and Opportunities in the Evolving Pharma Supply Chain Landscape
Despite the advancements and innovations in the pharmaceutical supply chain, several challenges persist, while new opportunities continue to emerge.
Here are key challenges facing the pharma supply chain and discuss strategies for overcoming these challenges while capitalising on emerging opportunities.
Challenges in the Pharma Supply Chain
– Regulatory Complexity: The pharmaceutical industry is subject to a complex web of regulatory requirements and compliance standards, which vary across different jurisdictions. Navigating regulatory complexities and ensuring compliance with evolving regulations can be challenging for pharmaceutical companies, particularly those operating in multiple markets.
– Supply Chain Disruptions: Global events, such as natural disasters, geopolitical tensions, and pandemics, can disrupt pharmaceutical supply chain operations, leading to shortages, delays, and supply chain bottlenecks. Building resilience and contingency planning are essential for mitigating the impact of supply chain disruptions and ensuring continuity of supply.
– Counterfeit Drugs and Product Safety: Counterfeit drugs pose a significant threat to patient safety and public health, undermining trust in the pharmaceutical supply chain. Pharmaceutical companies must implement robust authentication technologies, track & trace systems, and supply chain security measures to prevent counterfeit drugs from entering the supply chain.
– Cost Pressures and Margin Compression: Pharmaceutical companies face cost pressures from rising raw material costs, regulatory compliance expenses, and healthcare reimbursement constraints, leading to margin compression and profitability challenges. Optimising supply chain costs, improving operational efficiency, and exploring new revenue streams are critical for addressing cost pressures and maintaining competitiveness.
– Data Security and Cybersecurity Risks: With increasing digitalization and reliance on data-driven technologies, pharmaceutical companies are vulnerable to data security breaches and cybersecurity risks. Protecting sensitive supply chain data, intellectual property, and patient information from cyber threats requires robust cybersecurity measures, employee training, and risk management practices.
Opportunities for Innovation and Transformation
– Digital Transformation: Embracing digital technologies, such as blockchain, artificial intelligence (AI), and Internet of Things (IoT), presents opportunities for pharmaceutical companies to enhance supply chain visibility, traceability, and efficiency. Digital transformation initiatives can improve data accuracy, automate manual processes, and enable real-time decision-making to drive operational excellence.
– Personalized Medicine and Patient-Centric Care: The shift towards personalised medicine and patient-centric care models creates opportunities for pharmaceutical companies to develop targeted therapies, companion diagnostics, and value-added services tailored to individual patient needs. By leveraging data analytics, genomics, and patient engagement strategies, pharmaceutical companies can improve treatment outcomes, patient satisfaction, and adherence.
– Sustainable Supply Chain Practices: Adopting sustainable sourcing practices, eco-friendly packaging solutions, and renewable energy sources enables pharmaceutical companies to reduce environmental impact, enhance corporate social responsibility, and meet consumer demand for environmentally conscious products. Sustainability initiatives can drive cost savings, brand reputation, and stakeholder engagement while contributing to long-term sustainability goals.
– Collaborative Partnerships and Ecosystem Integration: Collaborating with supply chain partners, healthcare providers, and technology innovators fosters innovation, knowledge sharing, and ecosystem integration. Strategic partnerships enable pharmaceutical companies to access complementary capabilities, expand market reach, and accelerate product development, while ecosystem integration enhances supply chain visibility, resilience, and agility.
Supply Chain Resilience
Supply chain resilience refers to the ability of the pharmaceutical supply chain to anticipate, withstand, and recover from disruptions while maintaining continuity of supply and minimising impact on stakeholders. Building resilience requires proactive risk management, redundancy, flexibility, and collaboration among supply chain partners.
– Risk Identification and Assessment: Conducting comprehensive risk assessments and scenario planning helps identify potential threats, vulnerabilities, and interdependencies within the supply chain. Risk assessments evaluate factors such as geopolitical risks, natural disasters, regulatory changes, and supply chain disruptions to prioritise mitigation efforts and allocate resources effectively.
– Redundancy and Diversification: Introducing redundancy and diversification into the supply chain, such as multiple sourcing options, alternative suppliers, and redundant production capacity, mitigates single points of failure and reduces reliance on critical suppliers or geographic regions. Redundancy and diversification strategies enhance supply chain resilience by enabling rapid response to disruptions and minimising supply chain disruptions.
– Flexibility and Adaptability: Designing supply chain networks with built-in flexibility and adaptability enables stakeholders to respond quickly to changes in demand, supply, and market conditions. Flexible manufacturing processes, agile distribution networks, and responsive inventory management systems facilitate rapid adjustments and reallocation of resources to meet evolving customer needs and mitigate supply chain risks.
– Collaboration and Communication: Collaborative partnerships and open communication channels among supply chain partners enhance coordination, information sharing, and joint decision-making during disruptions. Establishing crisis management teams, conducting regular drills, and maintaining clear communication protocols enable stakeholders to coordinate response efforts, address challenges, and maintain operational continuity during crises.
Supply Chain Risk Management
Supply chain risk management involves identifying, assessing, prioritising, and mitigating risks that may impact supply chain operations, product quality, and customer satisfaction. Effective risk management practices help pharmaceutical companies anticipate and prepare for potential disruptions while minimising their impact on business performance and stakeholder interests.
– Risk Mitigation Strategies: Implementing risk mitigation strategies, such as supplier diversification, safety stock, inventory buffers, and contingency planning, helps reduce the likelihood and severity of supply chain disruptions. Risk mitigation measures address specific risks identified during risk assessments and prioritise actions to minimise potential impact on supply chain operations.
– Supply Chain Visibility and Transparency: Enhancing supply chain visibility and transparency enables stakeholders to monitor product movement, track inventory levels, and identify potential risks in real-time. Advanced analytics, track & trace technologies, and digital supply chain platforms provide stakeholders with actionable insights, enabling proactive risk management and timely response to disruptions.
– Business Continuity Planning: Developing robust business continuity plans (BCPs) and disaster recovery strategies ensures operational resilience and continuity of supply chain operations during disruptions. BCPs outline procedures for activating crisis management teams, reallocating resources, and implementing alternative strategies to maintain essential functions and minimise disruptions to customers.
– Continuous Improvement and Learning: Embracing a culture of continuous improvement and learning enables pharmaceutical companies to adapt to changing market conditions, emerging risks, and evolving regulatory requirements. Post-event analysis, root cause analysis, and lessons learned sessions help identify areas for improvement, strengthen risk management processes, and enhance organisational resilience over time.
Leveraging Emerging Technologies for Supply Chain Optimization
The pharmaceutical industry is witnessing a rapid evolution driven by technological advancements which can be used to optimise supply chain operations, improve efficiency, and enhance patient outcomes.
These include:
Artificial Intelligence and Machine Learning
Artificial intelligence (AI) and machine learning (ML) are revolutionising supply chain management by enabling predictive analytics, demand forecasting, and decision-making automation.
– Predictive Analytics: AI-powered predictive analytics algorithms analyse historical data, market trends, and external factors to forecast demand, identify supply chain risks, and optimize inventory levels. Predictive models enable stakeholders to anticipate demand fluctuations, mitigate stockouts, and optimize procurement and production schedules.
– Demand Forecasting: Machine learning algorithms analyze complex datasets and patterns to generate accurate demand forecasts, improving supply chain efficiency and reducing excess inventory. Advanced demand forecasting techniques leverage real-time data, customer behavior insights, and external factors to enhance forecast accuracy and responsiveness to market demand.
– Cognitive Automation: Cognitive automation technologies, such as robotic process automation (RPA) and natural language processing (NLP), automate routine tasks, streamline workflows, and improve decision-making processes. AI-driven chatbots, virtual assistants, and cognitive agents enhance supply chain collaboration, communication, and information sharing among stakeholders.
Internet of Things (IoT) and Sensor Technologies
The Internet of Things (IoT) and sensor technologies provide real-time visibility into supply chain operations, enabling stakeholders to monitor product conditions, track shipments, and optimize logistics processes.
– Condition Monitoring: IoT-enabled sensors monitor environmental conditions, such as temperature, humidity, and vibration, during transportation and storage of pharmaceutical products. Real-time data insights and alerts enable stakeholders to detect deviations from predefined thresholds, address issues proactively, and maintain product quality and integrity.
– Asset Tracking: RFID tags, GPS trackers, and Bluetooth beacons enable real-time tracking and tracing of pharmaceutical products, assets, and inventory throughout the supply chain. Asset tracking solutions enhance supply chain visibility, improve inventory accuracy, and reduce the risk of lost or stolen shipments.
– Cold Chain Management: IoT sensors and temperature monitoring devices play a crucial role in cold chain management, ensuring compliance with temperature-sensitive product requirements and regulatory standards. Cold chain monitoring solutions provide end-to-end visibility into temperature-controlled shipments, enabling stakeholders to maintain product quality and safety from manufacturing to delivery.
Blockchain Technology for Traceability and Transparency
Blockchain technology offers decentralised, tamper-proof ledger systems that provide transparent and traceable records of transactions and product movements across the supply chain.
– Track & Trace: Blockchain-based track & trace solutions enable stakeholders to trace the provenance of pharmaceutical products, verify authenticity, and ensure compliance with regulatory requirements. Immutable records stored on the blockchain provide a secure and auditable trail of product movements, facilitating rapid identification and resolution of supply chain issues.
– Supply Chain Transparency: Blockchain enhances supply chain transparency by providing stakeholders with real-time access to transparent and immutable records of transactions, inventory levels, and product movements. Transparent supply chain data enables stakeholders to collaborate more effectively, identify inefficiencies, and drive continuous improvement across the supply chain network.
Robotics and Automation in Manufacturing and Warehousing
Robotics and automation technologies streamline manufacturing processes, improve efficiency, and enhance safety in pharmaceutical manufacturing and warehousing operations.
– Automated Manufacturing: Robotic automation systems, such as robotic arms, automated guided vehicles (AGVs), and robotic dispensing systems, automate repetitive tasks, increase throughput, and ensure consistent product quality in pharmaceutical manufacturing facilities. Robotics enable flexible and agile manufacturing processes, allowing pharmaceutical companies to respond quickly to changing market demands and product requirements.
– Smart Warehousing: Automated warehouse systems, such as robotic palletizers, automated storage and retrieval systems (AS/RS), and autonomous mobile robots (AMRs), optimize warehouse operations, reduce labor costs, and improve inventory accuracy. Smart warehousing solutions leverage robotics, IoT sensors, and AI-driven algorithms to streamline order fulfillment, reduce picking errors, and enhance warehouse efficiency.
Regulatory Compliance and Quality Assurance in the Pharma Supply Chain
Compliance with regulatory requirements and adherence to quality standards are paramount in the pharmaceutical supply chain to ensure the safety, efficacy, and integrity of pharmaceutical products.
Regulatory Frameworks and Standards
The pharmaceutical industry is subject to a comprehensive regulatory framework designed to safeguard public health, ensure product quality, and maintain regulatory compliance. Regulatory agencies establish standards, guidelines, and requirements that govern various aspects of pharmaceutical manufacturing, distribution, and marketing.
– Good Manufacturing Practices (GMP): GMP regulations set forth quality standards for the manufacturing, processing, packaging, and storage of pharmaceutical products. GMP compliance ensures that pharmaceutical products are produced consistently, meet quality specifications, and are safe for use by patients.
– Good Distribution Practices (GDP): GDP regulations govern the distribution and transportation of pharmaceutical products, ensuring that products are handled, stored, and transported under appropriate conditions to maintain their quality, safety, and efficacy throughout the supply chain.
– Pharmacovigilance and Adverse Event Reporting: Pharmacovigilance regulations require pharmaceutical companies to monitor the safety of their products, report adverse events, and take appropriate measures to minimize risks to patients. Pharmacovigilance systems help identify and assess potential safety concerns associated with pharmaceutical products and facilitate timely regulatory interventions.
– Product Labeling and Packaging Requirements: Regulatory agencies prescribe labeling and packaging requirements to ensure that pharmaceutical products are properly identified, labeled, and packaged for safe and effective use by patients. Labeling regulations include requirements for product identification, dosage instructions, warnings, and precautions to inform healthcare professionals and patients about the risks and benefits of pharmaceutical products.
Quality Assurance and Control Measures
Quality assurance and control measures are essential for ensuring that pharmaceutical products meet quality specifications, regulatory requirements, and industry standards throughout the supply chain. Quality assurance encompasses proactive measures to prevent defects and ensure product quality, while quality control involves inspection and testing to detect and correct defects.
– Quality Management Systems (QMS): Pharmaceutical companies implement quality management systems to establish and maintain a culture of quality throughout the organization. QMS frameworks, such as ISO 9001, provide a structured approach to quality assurance, encompassing processes for document control, training, risk management, and continuous improvement.
– Batch Release and Quality Control Testing: Quality control laboratories perform batch release testing to verify the quality, safety, and efficacy of pharmaceutical products before they are released for distribution. Quality control tests include assays for potency, purity, identity, and dissolution, as well as tests for microbiological contamination and endotoxin levels to ensure product compliance with regulatory standards.
– Supplier Qualification and Audits: Pharmaceutical companies conduct supplier qualification audits to assess the capability, reliability, and compliance of suppliers and contract manufacturing organizations (CMOs). Supplier audits evaluate factors such as quality management systems, manufacturing processes, and adherence to regulatory requirements to ensure that suppliers meet quality standards and maintain product integrity.
– Continuous Improvement and Corrective Actions: Continuous improvement processes, such as CAPA (Corrective and Preventive Actions), enable pharmaceutical companies to identify root causes of quality issues, implement corrective actions, and prevent recurrence of deviations. CAPA systems promote a culture of continuous improvement, accountability, and transparency in addressing quality issues and enhancing product quality and compliance.
Regulatory Compliance Monitoring and Reporting
Pharmaceutical companies monitor and report compliance with regulatory requirements through various mechanisms, including regulatory inspections, audits, and submissions. Compliance monitoring and reporting processes ensure that pharmaceutical products meet regulatory standards and maintain regulatory approval for market authorization.
– Regulatory Inspections: Regulatory agencies conduct inspections of pharmaceutical manufacturing facilities, distribution centers, and supply chain partners to assess compliance with regulatory requirements, quality standards, and good manufacturing practices. Inspections may be scheduled or unannounced and cover various aspects of pharmaceutical operations, including facilities, processes, documentation, and personnel training.
– Regulatory Submissions and Approvals: Pharmaceutical companies submit regulatory filings, such as new drug applications (NDAs), abbreviated new drug applications (ANDAs), and marketing authorization applications (MAAs), to obtain regulatory approval for the marketing and distribution of pharmaceutical products. Regulatory submissions include comprehensive data on product safety, efficacy, quality, and manufacturing processes to demonstrate compliance with regulatory requirements and secure market authorization.
– Compliance Reporting and Documentation: Pharmaceutical companies maintain comprehensive documentation, records, and reports to demonstrate compliance with regulatory requirements and quality standards. Compliance reporting includes documentation of adverse events, product complaints, quality deviations, and corrective actions, as well as submission of periodic reports to regulatory agencies to provide updates on product safety, efficacy, and quality.
Future Trends and Opportunities
Looking ahead, the pharmaceutical supply chain is poised for continued evolution and transformation driven by technological advancements, regulatory developments, and changing market dynamics. Several key trends and opportunities are likely to shape the future of the pharmaceutical supply chain:
– Digital Transformation: The adoption of digital technologies such as artificial intelligence, Internet of Things, and blockchain will accelerate, enabling pharmaceutical companies to gain deeper insights, enhance collaboration, and improve decision-making across the supply chain.
– Personalised Medicine: The shift towards personalised medicine and precision therapy will drive demand for agile, patient-centric supply chain models that can accommodate individual treatment regimens, genetic variations, and therapeutic preferences.
– Regulatory Landscape: Regulatory requirements will continue to evolve, necessitating ongoing compliance efforts and investments in quality assurance, regulatory intelligence, and pharmacovigilance capabilities to navigate complex regulatory landscapes.
– Sustainability Initiatives: Environmental sustainability will become increasingly important, prompting pharmaceutical companies to invest in sustainable sourcing practices, green logistics, and renewable energy adoption to reduce carbon footprint and promote corporate social responsibility.
– Ethical Business Practices: Ethical considerations such as responsible sourcing, transparency, and stakeholder engagement will remain central to pharmaceutical supply chain operations, driving demand for ethical business practices and corporate social responsibility initiatives.