Ajinomoto Bio-Pharma Services is a fully integrated contract development and manufacturing organisation, with sites in Belgium, the United States, Japan, and India, providing comprehensive process development services, cGMP manufacturing, and drug product fill finish services of small molecule and large molecule active pharmaceutical ingredients (APIs) and intermediates.
Ajinomoto Bio-Pharma Services News
Description
Ajinomoto Bio-Pharma Services is a fully integrated contract development and manufacturing organisation (CDMO) with operations in Belgium, the United States, Japan, and India. It specialises in providing comprehensive development and cGMP manufacturing services for small and large molecule APIs and intermediates.
Key Products and Services
- Corynex® protein expression technology
- Oligonucleotide synthesis
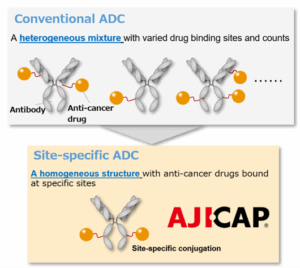
- Antibody-drug conjugations (ADC)
- High potency APIs (HPAPI)
- Biocatalysis and continuous flow manufacturing
- Aseptic fill-finish services
Ajinomoto Bio-Pharma Services is committed to innovation and delivering high-quality, client-focused solutions, supporting projects from pre-clinical development to commercial production.
Certification
United States FDA, Canada Health Canada, UK MHRA, Europe EMA, Belgium FAMHP, Japan PMDA, ISO 14001
Capabilities
- Accelerated Stability Studies
- Analytical Services
- APIs (Active Pharmaceutical Ingredients)
- Asceptic Fill-Finish
- Assay Development
- Bioanalytical Services
- Biocatalysis
- Bioequivalence
- Biologics
- Bioprocessing
- Biosimilars
- Cartridge
- Cell and Gene Therapy
- Cell Culture Media Development
- Chemistry (CMC)
- Chromatography
- Clean in Place / Sterilization in Place (CIP/SIP)
- Clinical Dose
- Clinical Packaging
- Clinical Trial Materials
- Clinical Trials
- Cold Chain Management
- Containment
- Contract Development and Manufacturing (CDMO)
- Controlled Substances
- Cytotoxic & High Potency Compounds
- Disposable/Single-Use
- Documentation Support Services
- Dosage Form Development
- Dose Form
- Downstream Processing
- Drug Delivery Devices
- Drug Development
- Feasibility Studies
- Fermentation
- Filling
- Finished Dosage Forms
- Forced Degradation Studies
- Formulation Development
- Glass
- High Containment Operations
- ICH
- Injectables
- Labelling
- Large Volume
- Liquids
- Lot Release Testing
- Lyophilization
- Microbial Fermentation
- Microbial-based Therapeutic Proteins
- Oligonucleotides
- Packaging
- Parenterals
- Parenterals Development
- Peptides
- Pilot-Scale Filling
- Preclinical Testing
- Preformulation
- Process Characterization
- Process Development
- Process Validation Studies
- Protein and Peptide
- Protein Extraction/Purification
- Proteins
- Purification
- Regulatory Services
- Scale-Up
- Solutions & Suspensions
- Stability Studies
- Sterile
- Sterile Filling
- Sterility Testing
- Sterilization
- Storage
- Supply Chain & Logistics
- Sustained Release
- Sustained Release Delivery Systems
- Syringe
- Technology Transfer
- Vaccines
- Validation
- Vials
- Warehousing
Contact Information
Website
Phone
Twitter (X)
Address
Cooppallaan 91
Zip/Post Code
9230
Facility Locations
India, United States, Belgium, Japan
Related Listings
UPCOMING EVENTS

CDMO Live Europe 2026
Experience the future of external manufacturing at CDMO Live, Rotterdam.
19-21st May 2026

External Manufacturing Leaders Boston 2026
Exclusive event for senior external manufacturing leaders. Boston, January 2026
RESOURCES
SPOTLIGHT