Clinical trial supply is a critical component of drug development, ensuring investigational medicinal products (IMPs) and materials reach research sites and patients with precision and compliance.
As the backbone of clinical research, effective supply chain management directly impacts trial timelines, data integrity, and ultimately, patient outcomes.
The global clinical trial supplies market was valued at approximately USD 2.73 billion in 2024 and is expected to grow at a CAGR of 7.19% from 2025 to 2030. This growth is largely fueled by rising R&D investments, the growing burden of chronic diseases, and the continued globalization of clinical trials. Additionally, the expanding focus on biologics and personalized medicine is increasing the need for advanced supply chain solutions, including temperature-controlled logistics and just-in-time delivery models. (Source- Grandview Research)

What is Clinical Trial Supply?
Clinical trial supply encompasses the end-to-end process of manufacturing, packaging, labeling, storing, and distributing investigational drugs and associated materials to clinical research sites and patients. This highly regulated supply chain ensures that:
- Investigational products maintain quality and integrity throughout their lifecycle
- Trial materials are delivered at the right time, to the right location, in the right condition
- Regulatory compliance is maintained across multiple jurisdictions
- Patient safety and data integrity are protected
Unlike commercial pharmaceutical supply chains, clinical trial supply must accommodate greater uncertainty, frequent protocol changes, and the unique challenges of handling investigational products that may have limited stability data.
Source- Akesa
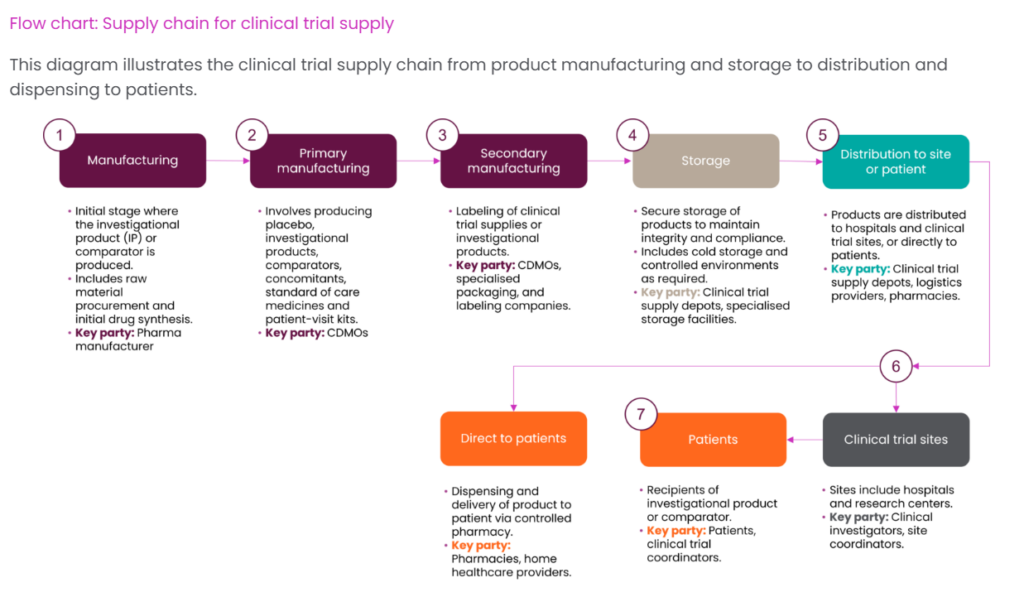
Key Components of the Clinical Trial Supply Chain
Manufacturing and Production
The clinical trial supply chain begins with the production of investigational medicinal products according to Good Manufacturing Practice (GMP) standards. This phase involves:
- Small-batch production: Specialized manufacturing of limited quantities
- Formulation development: Creating appropriate dosage forms for the clinical setting
- Analytical testing: Validating product quality, potency, and purity
- Documentation: Comprehensive batch records and certificates of analysis
Packaging and Labeling
Clinical trial materials require specialized packaging and labeling that:
- Maintains product stability and integrity
- Enables blinding procedures when required
- Complies with region-specific regulatory requirements
- Incorporates randomization codes and unique identifiers
- Provides clear instructions for proper handling and administration
Storage and Distribution
Clinical supplies must be stored and transported under carefully controlled conditions:
- Temperature monitoring: Continuous tracking for temperature-sensitive products
- Environmental controls: Protection from light, humidity, and other environmental factors
- Security measures: Prevention of unauthorized access or tampering
- Global logistics planning: Coordination across international borders and regulatory frameworks
Inventory Management
Effective inventory management ensures adequate supply while minimizing waste:
- Demand forecasting: Predicting site requirements based on enrollment projections
- Expiry tracking: Managing limited shelf-life of investigational products
- Resupply triggers: Automated systems for timely replenishment
- Returns processing: Accounting for unused or expired materials
Strategic Planning in Clinical Trial Supply
Successful clinical trial supply begins with comprehensive strategic planning that accounts for:
Risk Assessment and Mitigation
A robust risk management approach identifies potential disruption points:
- Supply chain vulnerabilities assessment
- Backup sourcing strategies development
- Alternative logistics pathways planning
- Quality deviation response protocols establishment
Demand Forecasting
Accurate forecasting balances availability against waste:
- Enrollment rate projections across sites
- Protocol-specific dosing requirements analysis
- Regional regulatory approval timeline considerations
- Buffer stock calculations based on variability factors
Regulatory Strategy
Navigating complex and varied regulatory landscapes requires:
- Country-specific importation requirements mapping
- Label translation and compliance planning
- Documentation preparation for multiple authorities
- Expiry extension strategies when appropriate
Regulatory Framework for Clinical Trial Supply
Clinical trial supply operates within a comprehensive regulatory framework designed to ensure patient safety and data integrity:
Global Regulatory Standards
Key regulatory guidelines that govern clinical trial supply include:
- Good Manufacturing Practice (GMP): Ensures product quality and consistency
- Good Distribution Practice (GDP): Maintains product integrity during transport
- Good Clinical Practice (GCP): Establishes overall trial conduct standards
- International Council for Harmonisation (ICH) guidelines: Provides harmonized approaches
Regional Regulatory Considerations
Regional variations create complexity in multi-country trials:
- FDA requirements in the United States
- EMA guidelines across European Union member states
- NMPA regulations in China
- PMDA standards in Japan
- Local import license requirements in emerging markets
Current Challenges in Clinical Trial Supply
Clinical trial supply is becoming increasingly complex due to adaptive trial designs, personalized treatments, and decentralized models that demand flexible and patient-centric solutions. Global supply chain disruptions—driven by pandemics, geopolitical issues, and raw material shortages—have further exposed vulnerabilities. Cold chain requirements for biologics and cell therapies add pressure with strict temperature controls and time-sensitive logistics. Rising costs from shipping, complex manufacturing, security, and regulatory compliance continue to strain trial budgets, making efficient and resilient supply strategies more critical than ever.
Innovations Transforming Clinical Trial Supply
Several technological advances are reshaping clinical trial supply:
Digital Supply Chain Solutions
Technology enables greater visibility and control:
- Interactive Response Technology (IRT): Automates randomization and supply management
- Blockchain applications: Enhance traceability and authenticity verification
- Predictive analytics: Improve forecasting accuracy and reduce waste
- Digital twin technology: Simulates supply chain scenarios for optimization
Direct-to-Patient Models
Decentralized approaches are gaining traction:
- Home delivery of investigational products
- Remote temperature monitoring of stored products
- Telehealth integration with supply logistics
- Patient-friendly packaging and administration systems
Sustainable Supply Chain Practices
Environmental considerations are increasingly important:
- Reduced packaging waste through innovative design
- Lower carbon footprint transportation strategies
- Energy-efficient storage facilities
- Recyclable and biodegradable materials adoption
Best Practices for Optimizing Clinical Trial Supply
- Integrated Planning: Involve supply chain early, align manufacturing with enrollment, and maintain clear site communication
- Smart Technology: Use real-time tracking, temperature monitoring, and analytics for end-to-end visibility
- Vendor Management: Partner with qualified vendors, define performance metrics, and conduct regular audits
- Contingency Plans: Prepare backups for sourcing, distribution, and manufacturing to handle disruptions smoothly
The Role of Outsourcing in Clinical Trial Supply
Many sponsors rely on specialized service providers to manage aspects of their clinical trial supply chain:
Benefits of Outsourcing
Strategic outsourcing can provide:
- Specialized expertise in complex logistics
- Geographic reach and local market knowledge
- Scalable capacity to accommodate fluctuating demand
- Cost efficiencies through shared resources and infrastructure
Types of Service Providers
The outsourcing landscape includes:
- Contract Manufacturing Organizations (CMOs): Focus on production of investigational products
- Clinical Supply Services Providers: Specialized in packaging, labeling, and distribution
- Integrated CDMOs: Offer end-to-end services from manufacturing through distribution
- Specialty Logistics Providers: Focus on complex transportation requirements
Effective Outsourcing Strategies
Maximizing outsourcing value requires:
- Clear definition of responsibilities and handoff points
- Transparent communication protocols
- Performance metrics aligned with trial objectives
- Quality oversight mechanisms and audit procedures
Key Considerations for Clinical Trial Supply Success
Successful trial supply starts with early integration into trial design—assessing supply risks, packaging needs, and distribution capabilities during protocol planning. Flexibility is vital, enabling quick adjustments for enrollment changes, amendments, or trial expansions.
Strong quality systems ensure compliance through documentation, audits, and deviation controls. Lastly, end-to-end visibility with real-time tracking and predictive analytics supports proactive decision-making and uninterrupted supply flow.
Conclusion
The clinical trial supply chain is a complex, highly regulated, and increasingly sophisticated ecosystem that directly impacts the success of drug development programs. As trials become more complex and global in scope, excellence in supply chain management has emerged as a competitive advantage that can accelerate timelines, control costs, and ultimately, bring new therapies to patients more efficiently.
Organizations that invest in strategic planning, technological innovation, and optimization of their clinical trial supply operations position themselves to navigate challenges more effectively and deliver more reliable outcomes. With continued evolution toward personalized medicine, decentralized trials, and global harmonization, the importance of supply chain excellence will only increase in the coming years.
Frequently Asked Questions
What is the difference between clinical supply and commercial supply?
Clinical supply involves smaller production volumes of investigational products under development, with frequent changes, complex logistics for blinded studies, and strict regulatory oversight. Commercial supply focuses on large-scale, standardized production of approved medications with established processes, more predictable demand forecasting, and stable supply networks primarily focused on efficiency and cost control.
What are clinical supplies in pharmaceutical manufacturing?
Clinical supplies include all materials needed to conduct clinical trials, primarily the investigational medicinal products being tested, but also comparator drugs, placebos, ancillary supplies (like syringes or administration devices), and supporting materials such as patient diaries, instruction sheets, and temperature monitoring devices.
How is clinical trial supply chain managed?
Clinical trial supply chain management involves strategic planning, manufacturing, packaging, labeling, storage, distribution, and inventory control of investigational products. It requires specialized systems like Interactive Response Technology (IRT), temperature monitoring solutions, quality management processes, and regulatory compliance frameworks across multiple jurisdictions, all coordinated through cross-functional teams that include clinical operations, manufacturing, logistics, and quality assurance.
What are the main challenges in clinical trial supply?
Key challenges include maintaining product integrity throughout the supply chain, managing temperature-sensitive products, navigating complex global regulatory requirements, accommodating protocol changes and enrollment fluctuations, ensuring blinding integrity, managing limited product shelf-life, controlling costs while maintaining quality, and adapting to emerging trial models like decentralized studies and personalized medicine approaches.
How are technology innovations improving clinical trial supply?
Technological innovations enhancing clinical trial supply include blockchain for traceability, IoT sensors for real-time temperature monitoring, AI and machine learning for demand forecasting, cloud-based supply chain management systems for global visibility, advanced analytics for optimization, automated inventory management solutions, mobile applications for site-level supply management, and direct-to-patient platforms supporting decentralized trials.
Recent Clinical Trial Supply News-
Pentixapharm Signs Manufacturing Deal with Eckert & Ziegler for Clinical Trial Supply (April 2025)
Eckert & Ziegler Expands Collaboration with GlyTherix to Supply Actinium-225 for Clinical Trials (Jan 2025)
Orano Med Doubles Clinical Supply Capacity at Plano Facility (June 2025)
Download our CDMO News Tracker to stay ahead of every shift in the CDMO landscape.








