Viral vector manufacturing represents the sophisticated process of producing modified viruses that serve as delivery vehicles for therapeutic genetic material.
These engineered vectors have revolutionised gene therapy applications, from treating rare genetic disorders to developing next-generation vaccines, including the COVID-19 adenoviral platforms that demonstrated the technology’s rapid deployment potential.
The viral vector manufacturing market has experienced unprecedented growth, reaching £3.8 billion in 2022 and projected to achieve £10.2 billion by 2028 at a robust CAGR of 18.2%. This explosive expansion reflects the increasing adoption of gene therapies and advanced vaccine platforms, positioning viral vector manufacturing as a critical capability in modern pharmaceutical development.
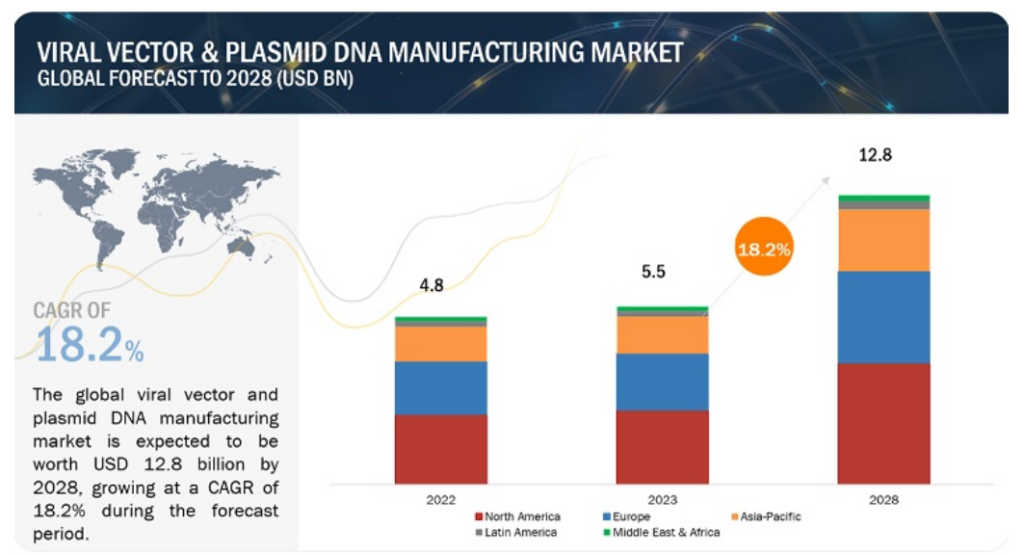
Source- Markets and Markets
What Is Viral Vector Manufacturing?
Viral vector manufacturing encompasses the end-to-end production of genetically modified viruses designed to deliver therapeutic genes to target cells. Unlike traditional small molecule manufacturing, this process requires specialised cell culture systems, complex purification protocols, and stringent quality control measures to ensure both safety and efficacy.
The manufacturing process transforms naturally occurring viruses into therapeutic delivery systems by removing pathogenic genetic elements whilst retaining the virus’s inherent ability to infect cells and deliver genetic cargo. This delicate balance between maintaining infectivity and ensuring safety represents one of the fundamental challenges in viral vector production.
Types of Viral Vectors in Pharmaceutical Manufacturing
Adeno-Associated Virus (AAV) Vectors
AAV vectors have emerged as the gold standard for many gene therapy applications due to their exceptional safety profile and tissue-specific targeting capabilities. These vectors demonstrate minimal immunogenicity and rarely integrate into the host genome, making them particularly suitable for treating genetic disorders affecting the liver, eye, and central nervous system.
Key characteristics:
- Non-pathogenic in humans
- Low immunogenic response
- Tissue-specific serotypes available
- Limited packaging capacity (~4.7 kb)
Adenoviral Vectors
Adenoviral vectors excel in vaccine applications and scenarios requiring high transduction efficiency. Their ability to infect both dividing and non-dividing cells, combined with strong immune responses, makes them valuable for immunotherapy and vaccine development.
Key characteristics:
- High transduction efficiency
- Strong immunogenic response
- Larger packaging capacity (~8 kb)
- Episomal expression (non-integrating)
Lentiviral Vectors
Derived from retroviruses, lentiviral vectors offer the unique advantage of genomic integration, providing sustained gene expression. This characteristic makes them particularly valuable for treating conditions requiring long-term or permanent genetic correction.
Key characteristics:
- Genomic integration capability
- Long-term gene expression
- Ability to transduce non-dividing cells
- Moderate packaging capacity (~9 kb)
The Viral Vector Manufacturing Process
Vector Design and Construction
The manufacturing process begins with careful vector design, where therapeutic genes replace pathogenic sequences within the viral genome. This step requires expertise in molecular biology and deep understanding of the target indication to optimise gene expression levels and duration.
Design considerations include:
- Promoter selection for tissue-specific expression
- Packaging constraints based on vector type
- Regulatory sequence optimisation
- Safety modifications to prevent replication
Cell Line Development and Maintenance
Producer cell lines serve as the foundation for viral vector manufacturing. These specialised cell lines, typically derived from human embryonic kidney (HEK293) or other mammalian sources, require careful characterisation and banking procedures to ensure consistent production.
Critical cell line attributes:
- Genetic stability over multiple passages
- Optimal growth characteristics in serum-free media
- Consistent viral vector yield and quality
- Absence of adventitious agents
Production and Harvest
Large-scale production occurs in controlled bioreactor systems, where producer cells are expanded and induced to generate viral vectors. The production phase typically involves:
- Cell expansion in appropriate culture media
- Transfection or infection to initiate vector production
- Harvest timing optimisation to maximise yield
- Primary recovery through clarification and concentration
Purification and Downstream Processing
Viral vector purification represents one of the most technically challenging aspects of manufacturing. The process must achieve high purity whilst maintaining vector infectivity and stability.
Common purification strategies:
- Ultracentrifugation for research-scale production
- Chromatography-based methods for commercial manufacturing
- Membrane filtration for concentration and buffer exchange
- Tangential flow filtration for scalable processing
Quality Control and Release Testing
Comprehensive analytical testing ensures viral vectors meet stringent safety and efficacy requirements. Quality control encompasses both product characterisation and safety testing.
Essential quality attributes:
- Vector genome titre (copies/mL)
- Infectious titre (functional units)
- Empty-to-full capsid ratio (for AAV)
- Residual host cell proteins and DNA
- Endotoxin levels
- Sterility and mycoplasma testing
Development Phase Manufacturing Considerations
Manufacturing requirements evolve significantly across development phases. Early-stage programmes from pre-clinical through Phase II focus primarily on process development, small-scale production capability, and analytical method establishment. The emphasis remains on rapid proof-of-concept activities and generating preliminary safety data with relatively modest manufacturing demands.
Late-stage development undergoes a fundamental shift toward commercial readiness. Phase III programmes require robust process performance qualification, comprehensive technology transfer planning, and detailed regulatory filing preparation. Supply chain establishment becomes critical, alongside continued process verification and commercial manufacturing capability validation.
Manufacturing Strategy: Make vs Buy Decisions
In-House Manufacturing Benefits
Pharmaceutical companies increasingly evaluate building internal viral vector capabilities to maintain control over their gene therapy programmes. Internal manufacturing offers several strategic advantages:
Operational control:
- Complete oversight of production timelines
- Proprietary process development
- Direct quality management
- Intellectual property protection
Economic considerations:
- Potentially lower cost of goods at commercial scale
- Reduced dependency on external capacity
- Long-term cost predictability
Contract Development and Manufacturing Organisation (CDMO) Advantages
Outsourcing to specialised CDMOs provides immediate access to established infrastructure and expertise, particularly valuable for smaller biotechnology companies or early-stage programmes.
Strategic benefits:
- Rapid programme initiation
- Access to regulatory expertise
- Reduced capital investment requirements
- Scalable capacity options
Practical considerations:
- CDMO booking timelines often extend 18+ months
- Technology transfer complexities
- Potentially higher variable costs
- Shared capacity constraints
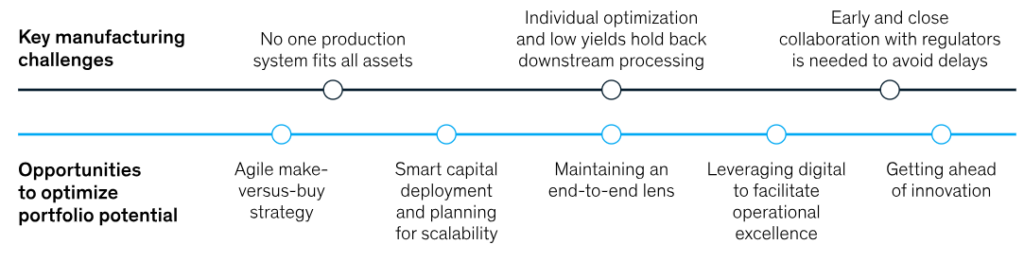
Source- McKinsey
Scalability Challenges and Solutions
Traditional Manufacturing Limitations
Conventional viral vector production methods face inherent scalability constraints that can impede commercial success:
- Adherent cell culture limitations in large-scale bioreactors
- Downstream processing bottlenecks in purification
- Quality control testing throughput constraints
- Regulatory compliance across multiple manufacturing scales
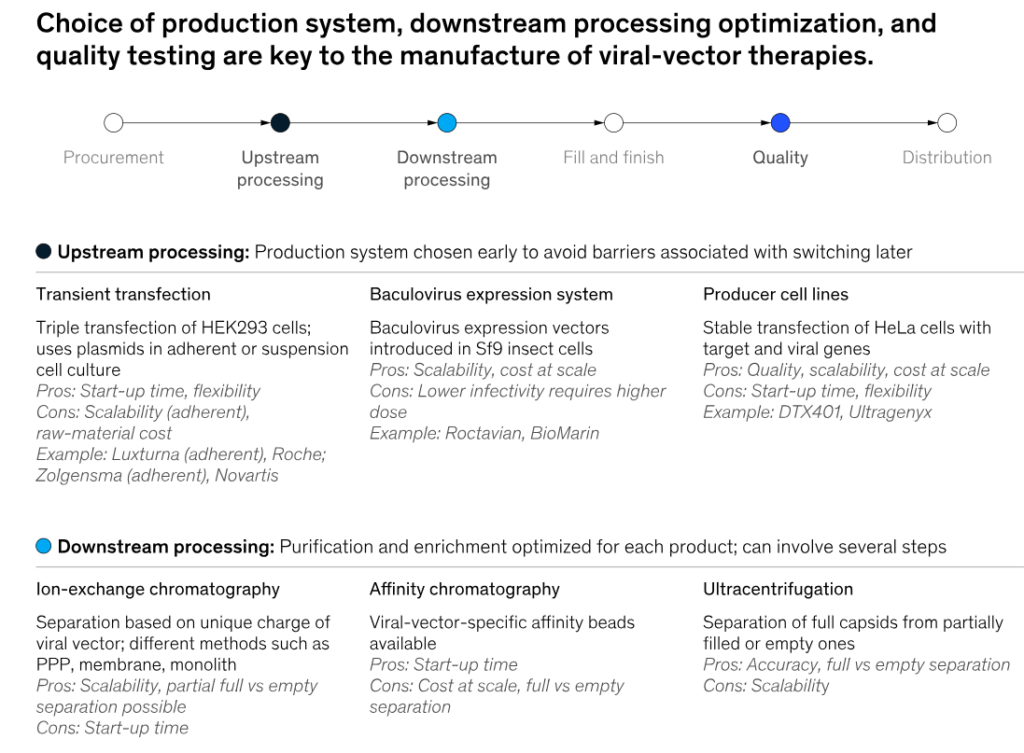
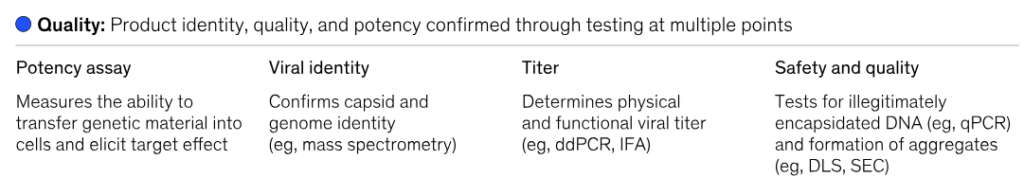
Source- McKinsey
Innovative Scalability Solutions
The industry has developed several approaches to address scalability challenges:
Process intensification:
- High-density cell culture systems
- Continuous manufacturing approaches
- Integrated upstream-downstream processing
Technology platforms:
- Suspension-adapted cell lines
- Single-use bioreactor systems
- Platform-based purification methods
Regulatory Considerations
Good Manufacturing Practice (GMP) Requirements
Viral vector manufacturing must comply with stringent GMP requirements throughout development and commercialisation. Key regulatory considerations include:
- Facility design with appropriate containment levels
- Personnel training and qualification programmes
- Cleaning validation for multi-product facilities
- Change control procedures for process modifications
International Regulatory Harmonisation
Regulatory requirements vary across global markets, requiring careful planning for international development programmes:
- European Medicines Agency (EMA) guidelines for gene therapy medicinal products
- FDA guidance on chemistry, manufacturing, and controls
- International Council for Harmonisation (ICH) quality guidelines
- Regional-specific requirements for emerging markets
Supply Chain and Storage Management
Viral vector manufacturing depends on specialised raw materials requiring careful supply chain orchestration. Plasmid DNA, often produced in-house to ensure quality and supply security, represents a critical component for transient transfection processes. Cell culture media and supplements must meet serum-free, chemically defined specifications, with lot-to-lot variability potentially impacting production consistency.
Ultra-low temperature storage requirements create unique logistical challenges throughout the supply chain. Viral vectors typically require storage at -80°C or below, necessitating robust cold chain logistics for distribution. Formulation development efforts focus on improving stability profiles, whilst specialised packaging systems maintain temperature integrity during transport. Storage stability studies determine shelf life parameters and guide commercial supply strategies.
Future Outlook and Innovation Trends
Emerging Technologies
Several technological innovations promise to transform viral vector manufacturing:
Next-generation production systems:
- Continuous manufacturing platforms
- Cell-free production systems
- Synthetic biology approaches
Enhanced purification methods:
- Affinity chromatography improvements
- Novel membrane technologies
- Integrated downstream processing
Market Evolution
The viral vector manufacturing landscape continues evolving rapidly:
- Capacity expansion driven by increasing demand
- Standardisation efforts across the industry
- Regulatory pathway maturation for gene therapies
- Cost reduction initiatives through process improvements
Key Takeaways-
Viral vector manufacturing represents a critical capability for pharmaceutical companies pursuing gene therapy development. Success requires careful consideration of manufacturing strategy, regulatory requirements, and technological capabilities.
Strategic imperatives:
- Early manufacturing strategy development aligned with product portfolio
- Robust quality systems implementation from programme inception
- Supply chain risk management for critical raw materials
- Regulatory engagement throughout development lifecycle
Operational excellence factors:
- Process development emphasis on scalability and robustness
- Comprehensive analytical method development and validation
- Technology transfer planning for commercial manufacturing
- Continuous improvement mindset for cost and quality optimisation
Frequently Asked Questions
What is viral vector manufacturing in the pharmaceutical industry?
Viral vector manufacturing is the process of producing genetically modified viruses that serve as delivery vehicles for therapeutic genes. These vectors are essential for gene therapy treatments and advanced vaccine development, requiring specialised production facilities and stringent quality controls.
What are the four main types of viral vectors used in pharma?
The primary viral vectors are Adeno-Associated Virus (AAV) vectors, adenoviral vectors, lentiviral vectors, and retroviral vectors. Each type offers distinct characteristics regarding safety profile, packaging capacity, and integration behaviour, making them suitable for different therapeutic applications.
How are viral vectors prepared for pharmaceutical use?
Viral vector preparation involves vector design and construction, producer cell line development, large-scale production in bioreactors, comprehensive purification through chromatography and filtration, and rigorous quality control testing to ensure safety, purity, and potency.
What are the main challenges in viral vector manufacturing?
Key challenges include scalability from laboratory to commercial production, maintaining product quality and consistency, managing complex supply chains for specialised raw materials, ensuring regulatory compliance across global markets, and addressing high manufacturing costs.
Should pharmaceutical companies manufacture viral vectors in-house or outsource?
The decision depends on factors including company size, product portfolio, development stage, and strategic objectives. In-house manufacturing offers greater control and potentially lower long-term costs, whilst outsourcing provides immediate access to expertise and established infrastructure with reduced capital investment.
What quality controls are essential for viral vector manufacturing?
Critical quality controls include vector genome titre determination, infectious titre measurement, empty-to-full capsid ratio assessment, residual impurity testing (host cell proteins and DNA), endotoxin testing, sterility verification, and comprehensive identity and purity analysis.
How long does viral vector manufacturing take from start to finish?
Production timelines vary significantly based on vector type and scale, but typically range from several weeks for small batches to 2-3 months for large-scale commercial production, including quality control testing and release procedures.
What regulatory approvals are needed for viral vector manufacturing?
Viral vector manufacturing requires compliance with Good Manufacturing Practice (GMP) regulations, facility licensing from relevant health authorities, and product-specific approvals as part of gene therapy marketing authorisations. Requirements vary by region and intended use.
Recent Viral Vector Manufacturing CDMO Announcements:
Minaris Partners with CGT Catapult to Advance Viral Vector Manufacturing Technologies (Dec 2025)
Oxford Biomedica Acquires Viral Vector Facility in North Carolina for $4.5M (October 2025)
DINAMIQS Opens Switzerland’s First cGMP Viral Vector Manufacturing Facility (October 2025)
Oxford Biomedica Acquires Viral Vector Facility in North Carolina for $4.5M (October 2025)
Matica Biotechnology Secures US Commercial Manufacturing Agreement for Viral Vectors (September 2025)
ViroCell Biologics Partners with AvenCell on Allogeneic CAR-T Manufacturing (July 2025)
ProBio Launches New Hopewell Centre to Expand Viral Vector Manufacturing (June 2025)
64x Bio Launches AAV Apex Suite to Tackle Gene Therapy Manufacturing Bottlenecks (June 2025)
Wacker Biotech Partners with Expression to Deliver Integrated Viral Vector Manufacturing (May 2025)
ViroCell Biologics Supports UCL Clinical Trial with High-Yield Lentiviral Vector (April 2025)
ChromaTan and Landmark Bio Secure NIIMBL Grant for AAV Manufacturing Innovation (March 2025)
Batavia Biosciences Partners with VBC and MEVAC to Boost Vaccine Manufacturing in Egypt (March 2025)
Novartis Opens €40M Viral Vector Facility in Slovenia to Boost Cell & Gene Therapy (February 2025)
Download our CDMO News Tracker to stay ahead of every shift in the CDMO landscape.








