Terminal sterilisation is a cornerstone of sterile drug product manufacturing. Recognised by regulatory authorities as the preferred sterilisation method, it offers unparalleled sterility assurance when applied appropriately.
The global terminal sterilisation services market is projected to grow from USD 13.09 billion in 2024 at a CAGR of 11.08% through 2030. This growth is driven by increasing surgical volumes, hospital-acquired infections, and demand for sterile drug manufacturing.
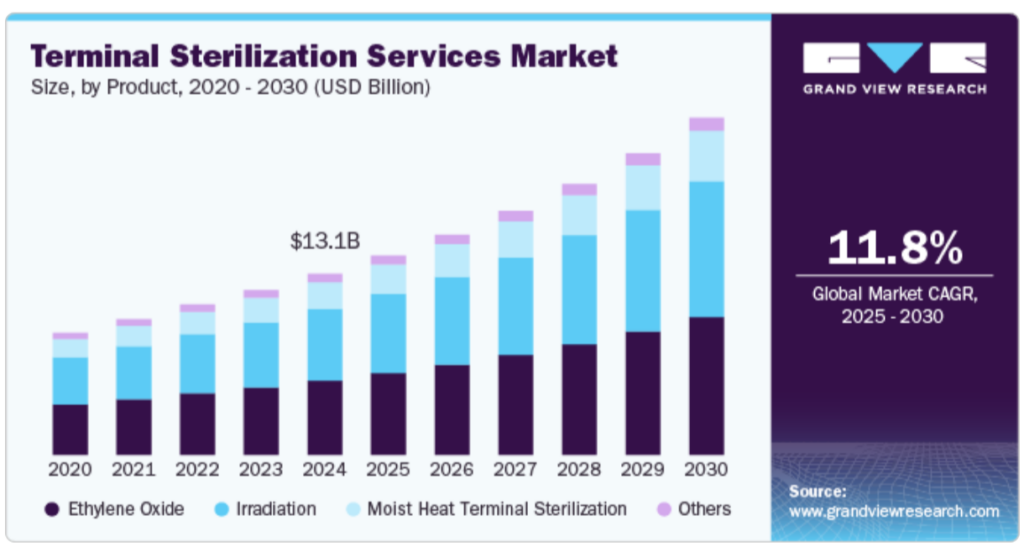
Source- Grandview Research
In this in-depth guide, we explore its definition, techniques, advantages, limitations, regulatory expectations, and strategic considerations for implementation in modern pharmaceutical manufacturing.
What Is Terminal Sterilisation?
Terminal sterilisation refers to the process of sterilising a product after it has been filled and sealed in its final container. Unlike aseptic processing, where sterility must be maintained throughout manufacturing, terminal sterilisation involves a validated sterilisation step that directly destroys all viable microorganisms.
Regulatory Consensus:
“Wherever possible, a process in which the product is sterilised in its final container (terminal sterilisation) is chosen.” — European Pharmacopoeia.
The FDA also advises that aseptic processing should be reserved for cases where terminal sterilisation is not feasible.
Key Benefits of Terminal Sterilisation
- High sterility assurance (SAL of ≤10⁻⁶)
- Reduced contamination risk post-sterilisation
- Lower cost of cleanroom operations
- Simplified process controls compared to aseptic filling
By sterilising sealed containers, this method eliminates potential for recontamination after the sterilisation step—a critical advantage in high-volume production environments.
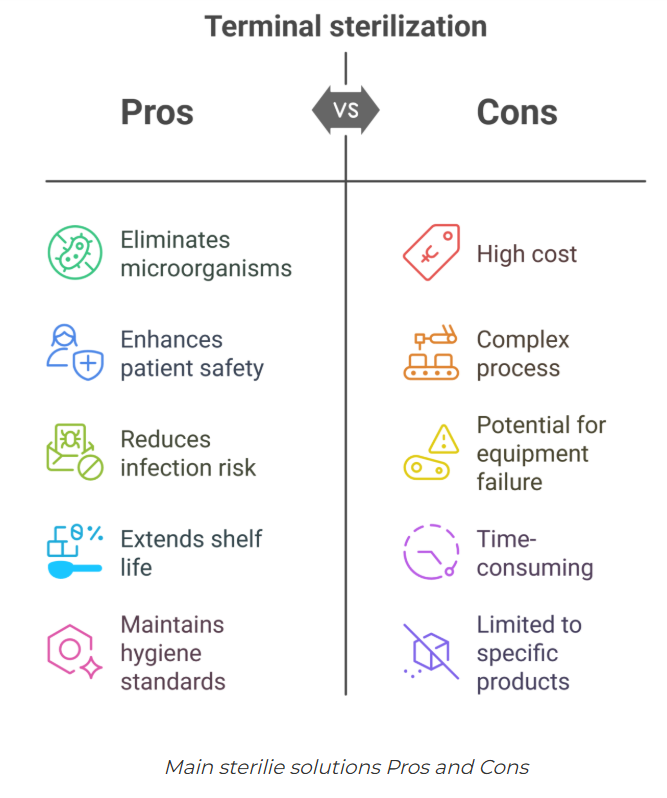
Source- Pharmuni
Common Applications in Pharma
Terminal sterilisation is most suitable for products and materials that can tolerate exposure to heat, radiation, or sterilising gases. Common examples include:
- Intravenous (IV) fluids such as saline, dextrose, or lactated Ringer’s
- Diluent solutions (e.g., water for injection, buffers, salts)
- Small molecule injectables that are heat-stable
- Parenteral solutions in glass or plastic containers
Why Terminal Sterilisation Is the Industry’s First Choice
Terminal sterilisation’s strength lies in its quantifiable SAL and reproducibility. For drug products that can withstand the conditions of the sterilisation method, terminal sterilisation not only simplifies environmental controls but also enhances product safety.
In many cases, manufacturers can avoid stringent environmental monitoring, redundant filtration, and complex aseptic validation activities—resulting in significant operational savings.
Key Sterilisation Methods Used in Terminal Processing
Each terminal sterilisation technique has distinct characteristics. Selecting the optimal method depends on product formulation, container material, and regulatory expectations.
1. Moist Heat (Steam) Sterilisation – Autoclaving
- Temperature: 121–134°C
- Cycle Time: 15–30 minutes
- Mechanism: Denatures proteins via steam penetration
- Best For: Aqueous solutions, glass containers, metals
Autoclaving is the most widely accepted terminal sterilisation method and generally preferred when product and container are compatible with heat and moisture.
Note: Packaging material sensitivity alone is not a valid reason to exclude steam sterilisation. Even 2 minutes at 121°C provides a meaningful sterility assurance level and may be suitable for many products.
2. Ethylene Oxide (EtO) Gas Sterilisation
- Best For: Heat- and moisture-sensitive products
- Mechanism: Alkylation of DNA/RNA in microorganisms
- Requirements: Pre-conditioning, exposure, and post-aeration
- Limitations: Residual gas toxicity; extended processing time
Ethylene oxide is highly penetrating and effective for complex devices or multi-material systems, but careful validation and safety measures are essential.
3. Radiation Sterilisation (Gamma or E-beam)
- Process: Ionising radiation damages microbial DNA
- Best For: Pre-packaged single-use devices
- Advantages: High throughput, no residuals
- Challenges: Potential material degradation, cost
Radiation offers a non-thermal option suitable for high-volume processing, though compatibility studies are essential.
4. Dry Heat Sterilisation
- Temperature: 160–180°C
- Cycle Time: 1–2 hours
- Best For: Oily preparations, powders, glassware
- Drawbacks: Long cycle times, energy intensive
Although less common, dry heat is essential for moisture-sensitive materials that can tolerate high temperatures.
5. Chemical Sterilisation (e.g., VHP, Peracetic Acid)
- Form: Vapour or liquid
- Best For: Equipment, containers not suitable for heat
- Considerations: Residuals, efficacy in presence of organic matter
Chemical sterilisation provides flexible alternatives but requires precise control and validation.
Regulatory Considerations and Industry Best Practices
Validating SAL
Terminal sterilisation cycles must be validated to demonstrate achievement of a SAL ≤10⁻⁶. This involves:
- Bioburden assessment and reduction strategy
- Biological indicator (BI) inactivation studies
- Product degradation and container closure integrity testing
Avoiding the “Overkill” Myth
A frequent misconception is that only overkill sterilisation cycles (e.g., 12-log reduction) are acceptable. However, for aseptically manufactured products with low bioburden, more moderate terminal cycles can suffice and should not be dismissed.
Even a sterilisation cycle with an F₀ of 2 is significantly better than skipping terminal sterilisation entirely.
Decision-Making Framework
A risk-based decision tree should be employed to evaluate terminal sterilisation feasibility early in development. Parameters to assess include:
- API and excipient stability under sterilisation conditions
- Packaging compatibility
- Bioburden control strategy
- Regulatory expectations (FDA, EMA, ICH)
When Is Terminal Sterilisation Not Feasible?
Some drug products cannot tolerate terminal sterilisation due to:
- Heat-sensitive active ingredients (e.g., monoclonal antibodies, peptides)
- Radiation-labile formulations
- Incompatibility with sterilising gases
In these cases, aseptic manufacturing with sterile filtration and stringent cleanroom control is required.
However, justification for not using terminal sterilisation must be supported by robust scientific evidence, not solely based on tradition or assumptions.
Cost, Efficiency, and Quality Considerations
Choosing terminal sterilisation can dramatically reduce operational complexity:
- Fewer cleanroom classifications and monitoring
- Reduced filtration validations
- Simplified environmental and personnel controls
Strategic benefit: Lower capital investment and reduced quality control overhead, especially in high-throughput settings.
Key Takeaway: Why Terminal Sterilisation Remains a Gold Standard
- Offers maximum sterility assurance through quantifiable and validated means
- Supported by regulators as the default method
- Enables more economical and simplified manufacturing processes
- Should always be considered first before aseptic processing alternatives
- Requires customised validation, not overkill, for product-friendly cycles
Frequently Asked Questions (FAQ)
Q1. What is terminal sterilisation in pharma manufacturing?
Terminal sterilisation is the final sterilisation of a sealed drug product container, using methods such as steam, EtO, radiation, or dry heat, to achieve a validated sterility assurance level.
Q2. How is terminal sterilisation different from aseptic processing?
Aseptic processing requires sterile conditions throughout, while terminal sterilisation sterilises the product after sealing. Terminal sterilisation offers a higher and measurable level of sterility assurance.
Q3. Why is terminal sterilisation preferred by regulators?
It minimises post-sterilisation contamination risk and provides a quantifiable SAL. Regulatory agencies require robust justification for not using it.
Q4. What products can be terminally sterilised?
IV fluids, saline, buffers, and heat-stable small molecules are commonly terminally sterilised. Biologics and heat-sensitive products typically are not.
Q5. Are overkill sterilisation conditions always required?
No. Overkill conditions (e.g., F₀ = 12) are not mandatory for low-bioburden products. Tailored cycles optimised for the product are acceptable.
Q6. What are the main terminal sterilisation methods?
The most common include steam (autoclave), ethylene oxide gas, radiation (gamma/e-beam), dry heat, and chemical sterilants like VHP.
Download our CDMO News Tracker to stay ahead of every shift in the CDMO landscape.








