An overview of APIs (Active Pharmaceutical Ingredients) and its significance in the pharmaceutical and biotech industries.
This guide provides you with the overall market size and trends, key drivers, opportunities and challenges, key suppliers in the category and how to improve strategic supplier partnerships.
API definition
The pharmaceutical and biotech industries heavily rely on APIs (Active Pharmaceutical Ingredients) for the development and production of drugs and therapies. APIs are the chemical substances responsible for the therapeutic effect of a pharmaceutical product. They are crucial components that provide the desired pharmacological activity.
The API market encompasses a wide range of chemical compounds that serve as active ingredients in pharmaceutical formulations. These compounds are crucial for delivering the desired therapeutic effect to patients. In this chapter, we provide an overview of the main product categories of APIs, highlighting their unique characteristics and applications.
APIs can be categorized based on various factors such as their chemical structure, therapeutic class, and mode of action. The following are some of the common product categories of APIs:
1. Small Molecule APIs: Small molecule APIs are organic compounds with a low molecular weight. They are chemically synthesized and account for a significant portion of the API market. These APIs are typically well-defined, stable, and have a high level of purity. Small molecule APIs are widely used in various therapeutic areas, including cardiovascular diseases, infectious diseases, oncology, and central nervous system disorders.
2. Biologic APIs: Biologic APIs, also known as large molecule APIs, are complex molecules derived from living organisms or manufactured through biotechnological processes. They include proteins, peptides, antibodies, and nucleic acids. Biologic APIs exhibit high specificity and are used in advanced therapies such as monoclonal antibodies, recombinant proteins, and gene therapies. They require specialized manufacturing processes and stringent quality control measures.
3. Synthetic APIs: Synthetic APIs are chemically synthesized compounds that are manufactured through organic synthesis. These APIs are created by assembling and modifying chemical structures using various reactions and techniques. Synthetic APIs offer advantages such as scalability, cost-effectiveness, and consistent quality. They are widely used in the pharmaceutical industry and have applications in diverse therapeutic areas.
4. Natural Product APIs: Natural product APIs are derived from natural sources such as plants, animals, or microorganisms. These APIs are obtained through extraction or isolation processes from natural materials. Natural product APIs have a long history of use in traditional medicine and continue to be valuable sources of therapeutic compounds. They often possess complex chemical structures and exhibit diverse pharmacological activities.
API Market Trends
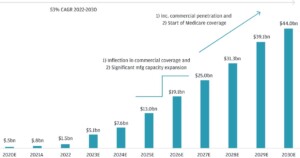
The global active pharmaceutical ingredient is forecast to reach $216.4 billion by 2027, growing at a CAGR of 8.2% from 2022 to 2027.
Market size value in 2022
$145.9 Billion (USD)
Revenue forecast 2027
$216.4 Billion (USD)
Growth Rate
8.2.% CAGR from 2022 to 2027
Source: Markets and Markets
Main product categories of APIs, along with examples and their applications:
- Cardiovascular APIs: These APIs are used in the treatment of cardiovascular diseases such as hypertension, angina, and heart failure. Examples include anti-hypertensive agents, anti-arrhythmics, and lipid-lowering drugs.
- Anti-Infective APIs: These APIs are utilized in the treatment of various infections caused by bacteria, viruses, fungi, and parasites. Antibiotics, antivirals, antifungals, and antiparasitic drugs are examples of anti-infective APIs.
- Oncology APIs: Oncology APIs are crucial in cancer treatment and include chemotherapeutic agents, targeted therapies, and immunotherapies. These APIs target cancer cells, inhibit their growth, and enhance the body’s immune response against tumors.
- Central Nervous System (CNS) APIs: APIs in this category are used in the treatment of neurological and psychiatric disorders. They include antidepressants, antipsychotics, anxiolytics, and analgesics.
- Hormonal APIs: Hormonal APIs are involved in regulating various physiological processes and are used in hormone replacement therapies, contraceptives, and treatments for hormonal imbalances.
- Gastrointestinal APIs: These APIs are used in the treatment of gastrointestinal disorders such as acid-related diseases, inflammatory bowel diseases, and gastrointestinal motility disorders.
- Respiratory APIs: Respiratory APIs are utilized in the treatment of respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), and allergic rhinitis.
- Dermatology APIs: Dermatology APIs are used in the treatment of various skin conditions and disorders. They include APIs for topical creams, ointments, and solutions used in the management of acne, eczema, psoriasis, and fungal infections.
- Ophthalmic APIs: Ophthalmic APIs are specifically formulated for ocular applications, including treatments for glaucoma, dry eye syndrome, and eye infections.
- Analgesic APIs: Analgesic APIs are used for pain management and relief. They include both non-opioid and opioid analgesics, providing different levels of pain control.
- Immunology APIs: Immunology APIs play a crucial role in modulating the immune system. They are used in the treatment of autoimmune diseases, allergies, and immunodeficiency disorders.
- Specialty APIs: Specialty APIs are unique and often complex molecules that target specific diseases or conditions. They include orphan drugs, personalized medicines, and advanced therapies, such as gene and cell therapies.
Market trends and key drivers
Several key drivers influence the dynamics of the API market. Understanding these drivers is essential for procurement executives to anticipate changes, capitalize on opportunities, and mitigate challenges. The following are some of the key drivers shaping the API industry:
1. Patent Expirations
The expiry of patents for blockbuster drugs creates opportunities for generic versions and biosimilars, leading to increased demand for APIs. Procurement teams must monitor patent expirations to identify potential API sourcing opportunities.
2. Emerging Markets
The pharmaceutical and biotech industries are experiencing rapid growth in emerging markets, particularly in Asia-Pacific regions. These markets offer significant opportunities for API manufacturers and procurement executives to expand their reach, tap into new customer bases, and leverage cost advantages.
3. Technological Advancements
Advancements in technology, such as process automation, data analytics, and artificial intelligence, are transforming the API manufacturing landscape. These technologies enhance efficiency, quality control, and supply chain transparency, allowing procurement teams to make data-driven decisions and optimize their sourcing strategies.
4. Personalised Medicine and Precision Therapies
The shift towards personalized medicine and precision therapies necessitates the development of APIs that target specific patient populations or genetic profiles. This trend drives the demand for specialty APIs and requires close collaboration between procurement teams and API manufacturers.
5. Increasing Focus on Sustainability
Environmental sustainability and green initiatives are gaining importance across industries, including pharmaceuticals. Procurement executives are increasingly seeking API manufacturers who prioritize sustainable practices, reduce environmental impact, and adhere to ethical and responsible sourcing.
SWOT Analysis
The API manufacturing market presents both significant opportunities and challenges for suppliers, as this SWOT analysis shows:
Strengths
Weaknesses
Strengths
- Established Supplier Networks: Procurement executives often have established supplier networks and relationships with reputable API manufacturers. These relationships provide a strong foundation for sourcing high-quality APIs and negotiating favourable terms and pricing agreements.
- Regulatory Compliance Expertise: Procurement teams possess expertise in navigating regulatory requirements and ensuring compliance throughout the supply chain. This strength is crucial in selecting API manufacturers that adhere to stringent quality standards, regulatory guidelines, and good manufacturing practices.
- Market Knowledge and Insight: Procurement executives have a deep understanding of the pharmaceutical market, including industry trends, product requirements, and customer needs. This knowledge enables them to identify potential API manufacturers, assess market dynamics, and make informed decisions that align with the organization’s goals and strategies.
Weaknesses
- Reliance on Limited Suppliers: Procurement teams may face the challenge of relying on a limited number of API suppliers, which can lead to reduced negotiation power and potential supply chain disruptions. Diversifying the supplier base and actively seeking new partnerships can help mitigate this weakness and ensure a more robust and resilient supply chain.
- Limited Transparency in the Supply Chain: Lack of transparency in the API supply chain can pose challenges in assessing the quality, sustainability, and ethical practices of API manufacturers. Strengthening supplier relationships, conducting thorough audits, and implementing traceability measures can address this weakness and ensure transparency and accountability throughout the supply chain.
Opportunities
Threats
Opportunities
- Increasing Demand for Specialty APIs: The growing demand for specialty APIs presents an opportunity for procurement executives to collaborate with manufacturers specializing in niche therapeutic areas. By partnering with specialized API manufacturers, procurement teams can ensure a reliable supply of high-quality APIs for targeted treatments and personalized medicine.
- Expansion in Emerging Markets: The rapid growth of pharmaceutical and biotech industries in emerging markets, particularly in Asia-Pacific regions, offers opportunities for procurement executives to explore new markets and establish strategic partnerships with API manufacturers in these regions. This expansion can provide cost advantages and access to a broader customer base.
- Advancements in Technology: Technological advancements in API manufacturing, such as process automation and data analytics, create opportunities for procurement executives to optimize supply chain management, improve efficiency, and enhance quality control. By leveraging these technologies and partnering with API manufacturers at the forefront of innovation, procurement teams can gain a competitive edge in the market.
Threats
- Supply Chain Disruptions: The API market is vulnerable to supply chain disruptions, including raw material shortages, manufacturing issues, geopolitical events, and natural disasters. These disruptions can impact the availability and pricing of APIs. Procurement teams must proactively monitor potential threats and develop contingency plans to mitigate risks and maintain a steady supply of APIs.
- Regulatory Changes: Changes in regulatory requirements and guidelines can pose challenges for procurement executives. New regulations may require additional documentation, testing, or compliance measures, which can impact the sourcing process and increase costs. Staying informed about regulatory updates and maintaining close communication with API manufacturers can help address potential threats and ensure compliance.
- Intense Market Competition: The API market is highly competitive, with numerous suppliers vying for market share. Intense competition can exert downward pressure on prices and pose challenges in securing reliable and cost-effective API sources. Procurement executives need to actively engage in supplier evaluation, negotiation, and continuous monitoring to ensure competitive pricing and maintain quality standards.
Building Strong Partnerships with API Manufacturers
To optimize partnerships with API manufacturers and ensure successful collaboration, pharmaceutical procurement teams should consider the following strategies:
Establish Clear Communication Channels
Effective communication is essential for building strong partnerships. Maintain regular and open communication with API manufacturers to discuss product requirements, timelines, and any potential challenges. Foster a collaborative environment where both parties can share insights and address issues promptly.
Develop Long-Term Relationships
Building long-term relationships with API manufacturers promotes stability and reliability in the supply chain. By fostering trust and understanding, procurement teams can gain preferential treatment, access to new products or technologies, and better pricing agreements.
Conduct Regular Audits and Inspections
Regular audits and inspections of API manufacturing facilities help ensure compliance with quality standards, regulatory requirements, and ethical practices. Procurement teams should prioritize manufacturers with robust quality management systems and track records of adherence to good manufacturing practices (GMP).
Encourage Innovation and Continuous Improvement
Engage with API manufacturers to encourage innovation and continuous improvement in manufacturing processes. Collaborate on research and development projects to explore new technologies, optimize efficiency, and enhance product quality. By fostering a culture of innovation, procurement teams can stay ahead of market trends and gain a competitive advantage.
Evaluate Total Cost of Ownership (TCO)
Consider the total cost of ownership when selecting API manufacturers. This includes not only the initial price of APIs but also factors such as transportation costs, packaging, storage requirements, and any potential hidden costs. A comprehensive evaluation of TCO helps in making informed decisions and identifying cost-saving opportunities.
Optimize Supply Chain Efficiency
Streamline the supply chain by identifying areas for efficiency improvement. Reduce lead times, minimize transportation costs, and optimize inventory management to eliminate unnecessary expenses. Collaborate closely with API manufacturers to implement lean supply chain practices and improve overall efficiency.
Negotiate Pricing Agreements
Engage in proactive negotiations with API manufacturers to secure competitive pricing agreements. Leverage market insights, competitive bids, and long-term commitments to negotiate favorable terms. However, it is crucial to balance cost considerations with the quality and reliability of the APIs.
Explore Alternative Sourcing Options
Diversify the sourcing strategy by exploring alternative API manufacturers and regions. This provides opportunities for cost savings, competitive pricing, and mitigating supply chain risks. However, ensure thorough evaluation of potential suppliers to maintain quality standards and regulatory compliance.
Implement Value Engineering
Collaborate with API manufacturers to identify opportunities for value engineering. Explore ways to optimize manufacturing processes, reduce waste, and enhance efficiency without compromising quality. Value engineering initiatives can help in cost reduction while maintaining product integrity.
Monitor Market Trends and Raw Material Prices
Stay updated on market trends and monitor the prices of raw materials used in API manufacturing. This information enables procurement teams to anticipate price fluctuations, assess the impact on API costs, and make informed sourcing decisions.
By implementing these strategies, pharmaceutical procurement teams can effectively manage costs without compromising the quality of APIs. Balancing cost considerations with quality, reliability, and regulatory compliance is crucial for maintaining a sustainable and efficient supply chain.