In the rapidly evolving biopharmaceutical landscape, biologics or ‘large molecule’ therapies have emerged as a crucial segment, promising innovative treatments for a wide-range of diseases.
90% of biotechs choose to partner with a Contract Development and Manufacturing Organization (CDMO) to get their new therapies through clinical trials and to market.
Biologics, derived from living organisms, require specialized development and manufacturing processes. This guide aims to equip biopharma decision-makers with the knowledge needed to select the right biologics CDMO partner, ensuring successful drug development and market entry.
What are Biologics?
In the biopharmaceutical industry, Biologics are advanced medicines created from living organisms or their components. Unlike traditional ‘small molecule‘ drugs made from chemicals, biologics include vaccines, gene therapies, and monoclonal antibodies.
These medicines are designed to target specific parts of the body, which makes them more effective and reduces side effects. For example, biologics can precisely attack cancer cells or modulate the immune system to treat autoimmune diseases. Their complexity and close relationship to human biology allow them to offer innovative treatments for conditions that were previously difficult to manage.
Definition and Sub-Categories of Biologics CDMOs
A Biologics CDMO is an external partner that offers comprehensive services in the development and manufacturing of biologic drugs. These services can encompass everything from initial development through to commercial manufacturing, including regulatory support, analytical testing, and packaging.
Subcategories of Biologics CDMOs:
- Mammalian Cell Culture CDMOs: Specialise in using mammalian cells to produce complex biologics such as monoclonal antibodies.
- Microbial Fermentation CDMOs: Focus on using bacteria or yeast for the production of biologics, including insulin and vaccines.
- Gene Therapy CDMOs: Provide services for the development and manufacturing of gene therapies, which involve modifying genetic material to treat diseases.
- Cell Therapy CDMOs: Specialise in the production of cell-based treatments, such as CAR-T therapies.
- Vaccine CDMOs: Focus on the development and manufacturing of vaccines for infectious diseases and other conditions.
How big is the small molecule CDMO market?
The biologics CDMO market was valued at USD 17.1 billion in 2024 and is projected to reach USD 39.0 billion by 2032, reflecting a CAGR of 11.0% from 2025 to 2032. Growth is driven by the rising prevalence of chronic diseases, increased outsourcing of R&D, expanding partnerships between biopharma companies and biologics CDMOs, and a growing geriatric population. (Source- P&S Intelligence)

Large pharmaceutical companies are increasingly outsourcing to reduce R&D risk, accelerate time-to-market for critical therapies, and lower manufacturing and development costs. At the same time, a growing number of biotech and specialty companies are turning to CDMOs to avoid the high fixed costs associated with in-house development, manufacturing, and specialized expertise.
As new molecular entities (NMEs) become more complex, demand is rising for niche technical capabilities and advanced competencies. Pharmaceutical companies increasingly prefer to access these capabilities externally through CDMOs rather than build them internally.
The infographic below summarises the biologics CDMOs operating in North America:

- Download the European CDMO infographic here and the North American CDMO infographic here
Key Trends in Biologics Contract Manufacturing:
The biologics contract manufacturing landscape is rapidly evolving, driven by technological advancements, regulatory changes, and market demands.
Trends such as single-use technologies, continuous manufacturing, digital and automation advancements, personalised medicine, and sustainability are shaping the future of the industry
1. Advancements in Single-Use Technologies (SUTs)
Single-use technologies are transforming biologics contract manufacturing. These disposable systems, used for tasks such as bioreactors and filtration, offer unparalleled flexibility and efficiency. By eliminating the need for cleaning and sterilisation between batches, SUTs reduce operational costs and downtime. Additionally, they significantly lower the risk of cross-contamination, making them ideal for producing high-quality biologics. SUTs also support scalability, easily adapting from small-scale clinical trials to large-scale commercial production, ensuring that CDMOs can meet diverse manufacturing demands.
2. Integration of Continuous Manufacturing
Continuous manufacturing is a revolutionary shift from traditional batch processing, bringing a host of benefits. This method involves a seamless, ongoing production process that enhances efficiency and throughput. Continuous manufacturing ensures consistent product quality through real-time monitoring and control. It also reduces production times and costs by minimising downtime and waste. Regulatory bodies like the FDA are increasingly endorsing continuous manufacturing for its potential to improve quality and efficiency in biologics production.
3. Adoption of Digital and Automation Technologies
The biologics contract manufacturing sector is embracing digital transformation and automation to improve efficiency and precision. Advanced data analytics enable better process understanding and optimization. Automated systems reduce human error and enhance productivity, while IoT devices facilitate real-time monitoring and proactive maintenance. Artificial intelligence applications, particularly in predictive analytics and quality
control, streamline operations and ensure high standards. These technologies collectively drive significant improvements in biologics manufacturing processes.
4. Focus on Personalized Medicine and Small Batch Production
The growing emphasis on personalized medicine is reshaping biologics manufacturing. Personalized therapies, such as CAR-T cell treatments, require bespoke production processes tailored to individual patients. This trend necessitates facilities capable of handling small, customized batches efficiently and cost-effectively. CDMOs are adapting to provide rapid turnaround times and maintain high quality, ensuring that personalized treatment protocols can be met. The ability to produce small batches without compromising on efficiency or quality is becoming increasingly important in the biologics sector.
5. Emphasis on Sustainability and Eco-friendly Practices
Sustainability is becoming a core focus for biologics contract manufacturers. Green manufacturing processes aim to reduce environmental impact through energy-efficient technologies and waste reduction. The use of recyclable and biodegradable materials in single-use systems and packaging is also on the rise. CDMOs are striving to meet stringent environmental regulations and adopt broader corporate social responsibility initiatives. These efforts not only support environmental sustainability but also enhance the overall reputation and compliance of biologics manufacturing operations.
The biologics external manufacturing panel at CDMO Live 2025, sponsored by BSP Pharmaceuticals, convened industry leaders to discuss the major challenges of scaling biologics production amid geopolitical pressures, capacity limitations, and shifting partnership models. The panel featured Olaf Birkenmeier, Head of External Manufacturing at Polpharma Biologics; Julien Laizé, Director of External Manufacturing CTM at Valneva; Catherine Seigneur, Senior Director of External Manufacturing Business Lead at UCB; and Giorgio Salciarini, Head of Sales – Technical Business Development at BSP Pharmaceuticals. Listen to the podcast here to hear the full discussion and insights shared during the session.

Services Offered by Biologics CDMOs:
Biologics Contract Development and Manufacturing Organizations provide a wide range of specialized services tailored to support the development, manufacturing, and commercialization of biologic drugs.
These services encompass every stage of the biologics lifecycle, from initial development through to final product distribution. Here’s a detailed view of the services you can expect from a biologics CDMO:
1. Development Services
Cell Line Development
- Description: Selection and optimization of host cell lines suitable for producing biologic drugs.
- Benefits: Ensures optimal productivity and stability of biologic production processes.
Process Development
- Description: Development and optimization of manufacturing processes to ensure efficient production of biologics.
- Benefits: Enhances yield, purity, and scalability of biologic drug manufacturing.
Analytical Method Development and Validation
- Description: Development and validation of analytical methods to assess the quality, potency, and purity of biologic drugs.
- Benefits: Ensures compliance with regulatory requirements and supports quality control throughout production.
2. Manufacturing Services
Upstream Manufacturing
- Description: Production of biologic drug substances using cell culture or microbial fermentation techniques.
- Benefits: Scalable production of biologics from laboratory to commercial scale.
Downstream Manufacturing
- Description: Purification and isolation of biologic drug substances from cell cultures or fermentation broths.
- Benefits: Removes impurities and concentrates biologic drugs to required potency levels.
Fill-Finish Services
- Description: Filling and packaging of biologic drug products into final dosage forms (e.g., vials, syringes).
- Benefits: Ensures sterile and safe delivery of biologic drugs to patients.
3. Quality Assurance and Control
GMP Compliance
- Description: Adherence to Good Manufacturing Practices (GMP) to ensure consistent quality, safety, and efficacy of biologic drugs.
- Benefits: Supports regulatory approval and market acceptance of biologic products.
Stability Testing
- Description: Evaluation of biologic drug stability under various environmental conditions (e.g., temperature, humidity).
- Benefits: Provides data on shelf-life and storage conditions for biologic products.
Batch Release Testing
- Description: Testing performed to verify the quality and compliance of each batch of biologic drugs before release to market.
- Benefits: Ensures that biologic products meet predefined specifications and regulatory requirements.
4. Regulatory Support
Regulatory Strategy and Submission Support
- Description: Development of regulatory strategies and preparation of documentation for regulatory submissions (e.g., IND, BLA).
- Benefits: Facilitates timely regulatory approval and market authorization of biologic drugs.
Inspections and Audits
- Description: Preparation for and management of regulatory inspections and audits by health authorities (e.g., FDA, EMA).
- Benefits: Ensures compliance with regulatory standards and guidelines.
5. Additional Services
Project Management
- Description: Coordination and management of biologics development and manufacturing projects from initiation to completion.
- Benefits: Ensures timelines, budgets, and quality standards are met throughout the project lifecycle.
Supply Chain Management
- Description: Management of biologic drug supply chains, including sourcing of raw materials and logistics.
- Benefits: Ensures reliable and efficient supply of biologic products to global markets.
Tech Transfer and Scale-Up
- Description: Transfer of biologic drug manufacturing processes between development and manufacturing facilities, and scaling production to commercial levels.
- Benefits: Facilitates seamless transition and scalability of biologic drug production.
Difference Between Biologics CMO, CRO, and CDMO
Understanding the differences between Biologics Contract Manufacturing Organizations (CMOs), Contract Research Organizations (CROs), and Contract Development and Manufacturing Organizations (CDMOs) is crucial in the pharmaceutical and biotech industries. Each plays a distinct role in drug development and production, focusing on different stages and aspects of the process.
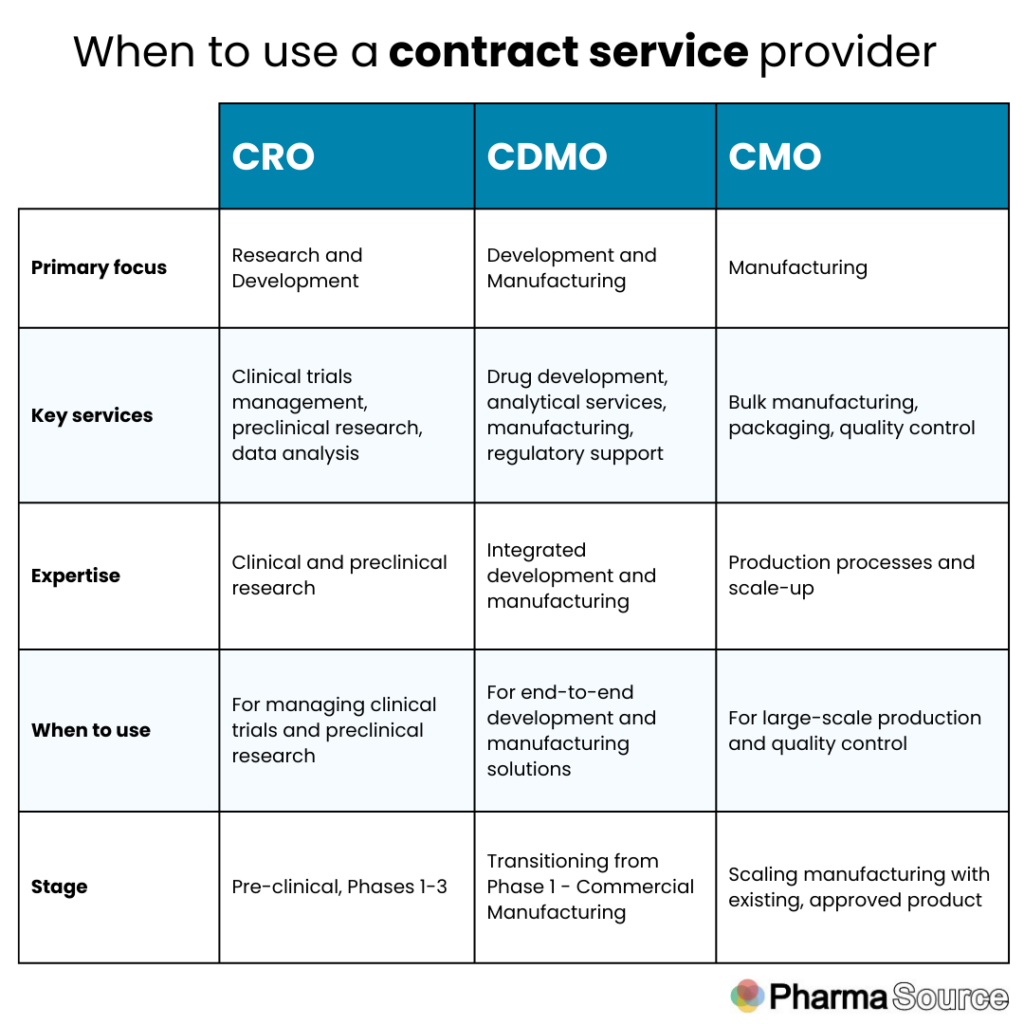
Here’s a detailed view of their differences:
Biologics CMO (Contract Manufacturing Organization)
Focus:
- Manufacturing: Biologics CMOs primarily focus on the manufacturing and production of biologic drugs.
Services:
- Upstream Manufacturing: Production of biologic drug substances using cell culture or microbial fermentation.
- Downstream Manufacturing: Purification and isolation of biologic drug substances.
- Fill-Finish Services: Filling and packaging of biologic drug products into final dosage forms.
Expertise:
- Technical Manufacturing Expertise: Specialized knowledge and infrastructure for large-scale biologics manufacturing.
- Quality Control: Ensuring adherence to Good Manufacturing Practices (GMP) and regulatory compliance.
Example:
- A biologics CMO might partner with a biotech company to scale up production of a monoclonal antibody for clinical trials or commercial distribution.
Contract Research Organization (CRO)
Focus:
- Research Services: CROs focus on providing research services to support drug discovery and development.
Services:
- Preclinical Research: Conducting non-clinical studies to evaluate safety and efficacy of drug candidates.
- Clinical Trials: Managing and conducting clinical trials to test investigational drugs in human subjects.
- Regulatory Support: Assisting with regulatory submissions and compliance.
Expertise:
- Scientific Expertise: High-level expertise in conducting research studies and clinical trials.
- Data Management: Handling and analyzing large volumes of clinical trial data.
Example:
- A CRO might assist a pharmaceutical company in conducting Phase II clinical trials for a new biologic drug candidate, from patient recruitment to data analysis.
Contract Development and Manufacturing Organization (CDMO)
Focus:
- End-to-End Services: CDMOs provide comprehensive services from drug development through to commercial manufacturing.
Services:
- Development: Services include cell line development, process optimization, and analytical method development.
- Manufacturing: Offering both development-scale and commercial-scale manufacturing capabilities.
- Regulatory Support: Assisting with regulatory strategy and submissions.
Expertise:
- Integrated Services: Seamlessly integrating development and manufacturing capabilities under one roof.
- Scale-Up Expertise: Ability to scale production from early-stage development to commercial manufacturing.
Example:
- A biologics CDMO might collaborate with a biopharmaceutical company from early-stage development of a new biologic therapy through to commercial manufacturing, providing expertise at every stage.
Summary
- Biologics CMOs specialize in the manufacturing of biologic drugs, focusing on large-scale production.
- CROs provide research services and manage clinical trials to support drug development.
- CDMOs offer integrated services from drug development through to commercial manufacturing, combining expertise in both development and production.
Choosing the right partner—whether a CMO, CRO, or CDMO—depends on the specific needs of the pharmaceutical or biotech company at each stage of drug development and commercialization, ensuring efficient and successful progression of biologic therapies from concept to market.
How to choose the right partner:
Choosing the right Biologics Contract Development and Manufacturing Organization (CDMO) is a critical decision that can significantly impact the success of your biologic drug development journey. Here’s how you can navigate this important choice in an engaging and effective manner:
1. Define Your Needs Clearly
Before diving into the selection process, clearly define your project requirements and goals. Consider aspects such as:
- Scale: Are you looking for small-scale development or large-scale commercial manufacturing?
- Expertise: Do you need specific expertise in cell line development, process optimization, or regulatory compliance?
- Timeline: What are your timelines for development, clinical trials, and market launch?
2. Assess Technical Capabilities
Look for a CDMO with robust technical capabilities that align with your project’s needs. Consider:
- Facilities: Evaluate their manufacturing facilities for cleanliness, scalability, and compliance with regulatory standards (e.g., GMP).
- Equipment: Ensure they have state-of-the-art equipment for both upstream (cell culture, fermentation) and downstream (purification, fill-finish) processes.
- Track Record: Review their track record in successfully handling projects similar to yours, including case studies and client testimonials.
3. Regulatory Compliance and Quality Assurance
Ensure the CDMO has a strong commitment to regulatory compliance and quality assurance:
- Regulatory Expertise: Verify their experience in navigating regulatory pathways (FDA, EMA, etc.) relevant to your geographic market.
- Quality Systems: Look for certifications (ISO, GMP) and quality systems that ensure consistent product quality and compliance.
4. Integrated Services and Flexibility
Consider CDMOs that offer integrated services and flexibility throughout the drug development lifecycle:
- End-to-End Services: Look for CDMOs that can support you from early-stage development through to commercial manufacturing, ensuring seamless transitions.
- Flexibility: Assess their ability to accommodate changes in project scope, timelines, and manufacturing scale as your project evolves.
5. Collaborative Culture and Communication
Choose a CDMO with a collaborative and communicative culture:
- Communication: Ensure clear communication channels and a responsive team that keeps you informed and involved throughout the project.
- Collaboration: Look for a partner who sees your success as their own, willing to adapt and innovate alongside your team.
6. Financial Stability and Long-Term Partnership
Evaluate the financial stability and reputation of the CDMO:
- Financial Health: Assess their financial stability and ability to invest in ongoing facility upgrades and technological advancements.
- Long-Term Partnership: Seek a CDMO committed to building a long-term partnership, offering strategic insights and support beyond initial project completion.
To keep up-to-date with the latest in biological contract manufacturing, join us at CDMO Live.
Recent Biologics CDMO News-
Sandoz to Acquire Evotec’s Just-Evotec Biologics EU SAS (Oct 2025)
Ajinomoto Bio-Pharma Services and Olon Partner to Scale Sustainable Biologics Manufacturing (Oct 2025)
Kemwell Biopharma Secures U.S. FDA Clearance for Commercial Biologics Manufacturing in Bengaluru (Oct 2025)
Aenova Expands Sterile Biologics Capacity with New Fill & Finish Line at Latina (Oct 2025)
INITS and FyoniBio Launch Strategic Collaboration for End-to-End Biologics CDMO Services (Oct 2025)
Alcami Expands Durham Laboratory by 20,000 sq. ft to Boost Biologics and CGT Capabilities (Oct 2025)
CR Gosun Launches Biologics Aseptic Fill-Finish Line in China (Oct 2025)
Lonza’s Stein Facility Gains Swissmedic Approval for Advanced Biologics Manufacturing (Oct 2025)
WuXi Biologics Launches TrueSite TI™ Platform to Accelerate Biologics Development (Sept 2025)
GSK commits $30 billion to US operations with new Pennsylvania biologics factory (Sept 2025)
Download our CDMO News Tracker to stay ahead of every shift in the CDMO landscape.
FAQs-
What is a biologics CDMO, and why do biotechs rely on them?
A biologics CDMO is a Contract Development and Manufacturing Organization that provides end-to-end support for biologic drug development, including cell line development, process optimization, GMP manufacturing, testing, and regulatory guidance. Around 90% of biotechs partner with CDMOs to access specialized expertise, reduce development risk, and accelerate their path through clinical trials and into the market.
How large is the biologics CDMO market, and what drives its growth?
The biologics CDMO market was valued at USD 17.1 billion in 2024 and is projected to reach USD 39.0 billion by 2032, growing at a CAGR of 11% from 2025–2032. Key growth drivers include rising chronic disease prevalence, increased outsourcing of R&D, complex biologic pipelines, and expanding biopharma–CDMO partnerships.
What types of biologics CDMOs operate in the industry?
Biologics CDMOs are typically categorized into mammalian cell culture CDMOs, microbial fermentation CDMOs, gene therapy CDMOs, cell therapy CDMOs, and vaccine CDMOs. Each provides specialized expertise for different classes of biologics, from monoclonal antibodies to CAR-T therapies and vaccines.
What services do biologics CDMOs typically offer?
Core services include cell line development, process development, upstream and downstream manufacturing, fill-finish, analytical testing, stability studies, GMP compliance, regulatory support, tech transfer, and supply chain management. These services support the full lifecycle of biologic drug development and commercialization.
What major trends are shaping biologics contract manufacturing?
Leading trends include the adoption of single-use technologies, continuous manufacturing, digital and automation advancements, personalized medicine requiring small-batch production, and increasing sustainability initiatives. These trends enhance efficiency, flexibility, and quality in biologics manufacturing.
How can biopharma companies choose the right biologics CDMO partner?
Companies should assess a CDMO’s technical capabilities, regulatory track record, facility quality, scalability, communication style, and financial stability. Priority should be given to partners offering integrated services, flexibility across development stages, and the ability to support long-term commercial manufacturing needs.








